A ratiometric fluorescent probe for rapid and sensitive visualization of hypochlorite in living cells†
Jiayu Zhaa,
Boqiao Fub,
Caiqin Qinb,
Lintao Zeng*ab and
Xichao Hu*b
aSchool of Chemistry & Chemical Engineering, Tianjin University of Technology, Tianjin 300384, PR China. E-mail: zlt1981@126.com; Fax: +86 22 60214252
bDepartment of Chemistry and Material Sciences, Hubei Engineering University, Hubei, Xiaogan 432000, PR China. E-mail: hxc30@163.com; Fax: +86 712 2345265
First published on 26th August 2014
Abstract
A ratiometric fluorescent probe for ClO− has been developed based on coumarin–hemicyanine, which displayed a colorimetric and fluorescence response to ClO− with high selectivity, fast response (within 2 min) and an extremely low detection limit (0.08 μM). This probe was successfully used to visualize hypochlorite in living cells.
Hypochlorite anion (ClO−)/hypochlorous acid (HOCl), a biologically important reactive oxygen species (ROS), is generally produced in living organisms from the myeloperoxidase (MPO)-mediated peroxidation of chloride ions and hydrogen peroxide.1 The hypochlorite anion (ClO−) plays a key role in the human immune defense system and inflammation by destruction of the invading bacteria and pathogens.2 However, excessive or misplaced production of ClO− can lead to tissue damage and diseases, such as atherosclerosis,3 arthritis,4 cancer,1b and neurodegeneration.5 Therefore, it is of great importance to investigate the complex contributions of HOCl/ClO− to our health and study the mechanism of action and specific functions of HOCl in living organisms. Scientists have conducted extensive research to elucidate the mechanism by which HOCl kills bacteria and destroys human tissue.6 However, the detailed understanding of HOCl formation during pathogenic biological events still remains a challenge due to the lack of methods for monitoring HOCl in living organisms.7
Fluorescence-based assays are useful tools for real-time sensing and for visualizing some biologically important species in living organisms because of their high sensitivity, and high temporal and spatial resolution.8 Several fluorescent probes for the detection and visualization of HOCl in living cells have been developed on the basis of the HOCl-mediated oxidation reaction of various functional groups such as p-methoxyphenol,9 ether,10 thioether,11 oxime,12 and hydroxamic acid.13 However, most of these probes just show changes in emission intensity, which are usually influenced by environmental conditions, probe distribution, and instrumental efficiency.14 By contrast, a ratiometric probe which utilizes the ratio of two emissions at different wavelengths as the detecting signal,15 can provide a built-in correction for the above mentioned factors and thus allow more accurate analysis. The fluorescence resonance energy transfer (FRET) mechanism provides an effective approach for construction of a ratiometric fluorescent probe. This strategy has been successfully employed to design a few ratiometric fluorescent probes for ClO− and has achieved good performance.16,17 Nevertheless, it is difficult to construct such a system because of the complicated synthetic routes as well as the requirement for strong spectral overlap between emission of donor and absorption of acceptor. Thus, it is highly desirable to develop a ratiometric probe for ClO− based on a simple and efficient approach.
Diethylamino-coumarin dye possesses several favourable fluorescence properties, such as excitation and emission wavelengths in the visible region as well as a high fluorescence quantum yield, which are desirable for the intended biological applications of the probe. In this work, we chose diethylamino-coumarin aldehyde as one building block, and then modified it by 1,2,3,3-tetramethyl-3H-indolium iodide to produce a water soluble CMCY with long emission wavelength (shown in Scheme 1). The structure of CMCY was confirmed by 1H NMR, 13C NMR and HR-MS. It is well known that hypochlorite anion (ClO−)/hypochlorous acid (HOCl) have strong reactivity to double bonds. We expect that ClO− will react with the double bonds and destroy the large π-conjugation of the probe, giving rise to a colorimetric and ratiometric fluorescence response to ClO−. This hybrid coumarin–hemicyanine probe exhibited an emission maximum at 631 nm. Upon addition of ClO−, the colour of the resultant solution changed from purple to colourless and the fluorescence of the solution changed from red to blue.
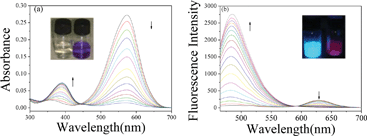
To examine the response of CMCY to ClO−, UV-vis absorption spectra titrations were performed in PBS/MeOH (pH 7.4, v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at ambient temperature. As shown in Fig. 1, free probe displayed a strong absorption band centred at 570 nm. Upon addition of ClO−, the absorption band centred at 570 nm gradually decreased and a new absorption band at 392 nm emerged with a well-defined isosbestic point at 455 nm. As a result, an obvious colour change from purple to colourless was observed, allowing colorimetric detection of ClO− by the naked eye (Fig. 1a inset). When the amount of ClO− reached 20 equivalents with respect to the probe, these changes were found to reach a plateau. A linear calibration of the UV-vis absorption response to ClO− concentrations from 0 to 73 μM was obtained (Fig. 2a), indicating that CMCY could be potentially used to quantitatively detect ClO− concentrations. We further examined the time course of the ratiometric response of CMCY to ClO− (Fig. 3a). CMCY was highly stable under the assay conditions. However, upon addition of 200 μM ClO−, a dramatic colour change from purple to colourless and a large increase in the ratio were observed within 2 min. This finding suggested that CMCY was a fast-response probe for ClO− and might be suitable for real-time sensing of ClO− in living cells.
1) at ambient temperature. As shown in Fig. 1, free probe displayed a strong absorption band centred at 570 nm. Upon addition of ClO−, the absorption band centred at 570 nm gradually decreased and a new absorption band at 392 nm emerged with a well-defined isosbestic point at 455 nm. As a result, an obvious colour change from purple to colourless was observed, allowing colorimetric detection of ClO− by the naked eye (Fig. 1a inset). When the amount of ClO− reached 20 equivalents with respect to the probe, these changes were found to reach a plateau. A linear calibration of the UV-vis absorption response to ClO− concentrations from 0 to 73 μM was obtained (Fig. 2a), indicating that CMCY could be potentially used to quantitatively detect ClO− concentrations. We further examined the time course of the ratiometric response of CMCY to ClO− (Fig. 3a). CMCY was highly stable under the assay conditions. However, upon addition of 200 μM ClO−, a dramatic colour change from purple to colourless and a large increase in the ratio were observed within 2 min. This finding suggested that CMCY was a fast-response probe for ClO− and might be suitable for real-time sensing of ClO− in living cells.
As shown in Fig. 1b, CMCY displayed a characteristic emission band centred at 631 nm. Upon addition of increasing amounts of ClO−, the fluorescence intensity at 631 nm gradually decreased and this was accompanied by the appearance of a new emission band centred at 480 nm. Consequently, the fluorescence of CMCY solution changed from red to blue. In the presence of 20 equiv. ClO−, the ratio of emission intensity (I480/I631) showed a drastic enhancement (ca. 400-fold enhancement), establishing that CMCY could serve as a ratiometric fluorescent probe for ClO−. It is worth noting that such a huge change of signal ratio at two wavelengths is highly desirable for ratiometric fluorescent probes, as the sensitivity and the dynamic range of ratiometric probes are controlled by the ratios. By plotting the ratio of emission intensity (I480/I631) versus the concentration of ClO−, a good linear relationship (R2 = 0.9932) was obtained with ClO− concentrations ranging from 0 to 73 μM (shown in Fig. 2b). The detection limit of probe CMCY for ClO− was determined to be 0.08 μM according to the signal-to-noise ratio (S/N = 3), which is comparable to the previously reported ratiometric fluorescent probes for ClO−.16,18
The ratiometric fluorescence responses of CMCY to ClO− at different pH values were also investigated, as shown in Fig. 3b. In the absence of ClO−, CMCY did not display any fluorescence changes over a wide range of pH values from 4.0 to 7.8. However, in the presence of 200 μM ClO−, a very large ratiometric fluorescence enhancement was observed from pH 6.5 to 7.8, which indicated that CMCY would be suitable for bio-applications at physiological conditions.
Good selectivity is a crucial requirement for all kinds of detection methods. To test the selectivity, CMCY (10 μM) was incubated with 10 equiv. of representative species including ROS, anions and ions. As shown in Fig. 4, a large ratiometric signal with I480/I631 = 15.7 was observed in the presence of 10 equiv. of ClO−. By contrast, other ROS (hydrogen peroxide, hydroxyl radical, superoxide, t-BuOOH and ONOO−) induced a very weak ratiometric response with I480/I631 < 0.5. Some common anions (CH3COO−, F−, ClO4−, PO43−, HCO3−, NO3−, SO32−, NO2− and SO42−) and heavy metal ions (Hg2+, Cu2+) caused only negligible changes in the emission ratio (I480/I631 < 0.2). These results suggest that the probe has excellent selectivity for ClO− over other anions and ROS. Although ozone is a very strong oxidation reagent and can break alkene to give aldehyde and carboxylic acid, it also easily oxidizes many biological species including GSH, protein and antibody. As we known, the content of GSH, protein and antibody are very rich in living cells. As a result, ozone has been largely consumed by these biological species, and thus will cause little interference to our probe. Therefore, the probe CMCY has potential applications for ClO− detection in complex biological environments.
To get an insight into the sensing mechanism, CMCY was treated with 20 equiv. ClO− in PBS for 30 min, and then the major fluorescent product was separated by silica gel column for MS analysis. The ESI-MS (+mode) showed the presence of a dominant peak at m/z = 358.0976 and a noticeable isotopic peak at 360.0947, which corresponded to a coumarin derivative shown in Scheme 2 and Fig. S2.† Hence, spectral changes in the presence of ClO− originated from the oxidative cleavage of CMCY by ClO−.
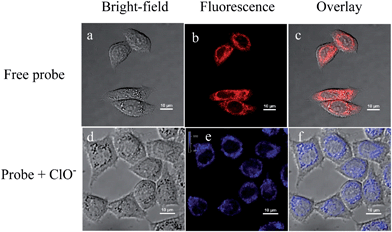
Furthermore, we explored the potential application of CMCY for sensing ClO− in living cells. HeLa cells incubated with CMCY (5 μM) for 30 min exhibited a clear cell profile with red colour (Fig. 5b), which indicated that CMCY has penetrated into the cells and been retained. When the HeLa cells were further treated with 100 μM ClO− for 30 minutes, blue fluorescence from the HeLa cells was observed (shown in Fig. 5e). Meanwhile, the original red fluorescence from CMCY was not detected, implying that CMCY had been consumed by ClO− and produced some products with blue fluorescence. Cell staining results indicated that probe CMCY was cell membrane permeable and capable of responding to ClO− in the living cells.
In summary, we have designed a water-soluble red-emitting probe based on coumarin–hemicyanine, which displayed a noticeable colorimetric and fluorometric dual response to ClO− on the basis of a simple alkene cleavage reaction. CMCY exhibited a fast response to ClO− in physiological conditions with high sensitivity and selectivity, and an extremely low detection limit (0.08 μM). By plotting the ratio of emission intensity (I480/I631) versus the concentration of ClO−, a good linear relationship (R2 = 0.9932) was obtained with ClO− concentrations ranging from 0 to 73 μM. Cell staining results indicated CMCY was cell membrane permeable and could be used for visualizing hypochlorite in living cells.
Acknowledgements
This work was financially supported by NSFC (no. 21203138, 31371750), the Natural Science Foundation of Tianjin (13JCQNJC05300), Hubei Provincial Educational Department (D20132702), and Hubei Co-Innovation Center for Utilization of Biomass Waste.Notes and references
- (a) Y. W. Yap, M. Whiteman and N. S. Cheung, Cell. Signalling, 2007, 19, 219 CrossRef CAS PubMed; (b) D. I. Pattison and M. J. Davies, Biochemistry, 2006, 45, 8152 CrossRef CAS PubMed.
- (a) D. Roos and C. C. Winterbourn, Science, 2002, 296, 669 CrossRef CAS PubMed; (b) F. C. Fang, Nat. Rev. Microbiol., 2004, 2, 820 CrossRef CAS PubMed.
- S. Sugiyama, K. Kugiyama, M. Aikawa, S. Nakamura, H. Ogawa and P. Libby, Arterioscler., Thromb., Vasc. Biol., 2004, 24, 1309 CrossRef CAS PubMed.
- M. J. Steinbeck, L. J. Nesti, P. F. Sharkey and J. J. Parvizi, J. Orthop. Res., 2007, 25, 1128 CrossRef CAS PubMed.
- D. I. Pattison and M. J. Davies, Chem. Res. Toxicol., 2001, 14, 1453 CrossRef CAS PubMed.
- (a) M. D. Rees, C. L. Hawkins and M. J. Davies, J. Am. Chem. Soc., 2003, 125, 13719 CrossRef CAS PubMed; (b) L. J. Marnett, J. Org. Chem., 2012, 77, 5224 CrossRef CAS PubMed; (c) J. Park, H. Kim, Y. Choi and Y. Kim, Analyst, 2013, 138, 3368 RSC.
- K. Setsukinai, Y. Urano, K. Kakinuma, H. J. Majima and T. Nagano, J. Biol. Chem., 2003, 278, 3170 CrossRef CAS PubMed.
- (a) J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973 CrossRef CAS PubMed; (b) Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192 CrossRef CAS PubMed; (c) L. Zeng, N. Fan, J. Zha, X. Hu, B. Fu, C. Qin and L. Wang, Analyst, 2013, 138, 7083 RSC.
- Z.-N. Sun, F.-Q. Liu, Y. Chen, P. K. H. Tam and D. Yang, Org. Lett., 2008, 10, 2171 CrossRef CAS PubMed.
- J. Shepherd, S. A. Hilderbrand, P. Waternan, J. W. Heinecke, R. Weissleder and P. Libby, Chem. Biol., 2007, 14, 1221 CrossRef CAS PubMed.
- (a) S. Kenmoku, Y. Urano, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 7313 CrossRef CAS PubMed; (b) Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 5680 CrossRef CAS PubMed.
- (a) W. Lin, L. Long, B. Chen and W. Tan, Chem.–Eur. J., 2009, 15, 2305 CrossRef CAS PubMed; (b) X. Cheng, H. Jia, T. Long, J. Feng, J. Qin and Z. Li, Chem. Commun., 2011, 47, 11978 RSC; (c) G. Cheng, J. Fan, W. Sun, K. Sui, X. Jin, J. Wang and X. Peng, Analyst, 2013, 138, 6091 RSC.
- Y.-K. Yang, H. J. Cho, J. Lee, I. Shin and J. Tae, Org. Lett., 2009, 11, 859 CrossRef CAS PubMed.
- (a) K. Komatsu, Y. Urano, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 13447 CrossRef CAS PubMed; (b) D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596 CrossRef CAS PubMed; (c) L. Long, D. Zhang, X. Li, J. Zhang, C. Zhang and L. Zhou, Anal. Chim. Acta, 2013, 775, 100 CrossRef CAS PubMed; (d) Y. Zhou, J. Y. Li, K. H. Chu, K. Liu and C. Yao, Chem. Commun., 2012, 48, 4677 RSC.
- J. V. Mello and N. S. Finney, Angew. Chem., Int. Ed., 2001, 40, 1536 CrossRef CAS.
- (a) L. Yuan, W. Lin, J. Song and Y. Yang, Chem. Commun., 2011, 47, 12691 RSC; (b) L. Yuan, W. Lin, Y. Xie, B. Chen and J. Song, Chem.–Eur. J., 2012, 18, 2700 CrossRef CAS PubMed.
- (a) X. Wu, Z. Li, L. Yang, J. Han and S. Han, Chem. Sci., 2013, 4, 460 RSC; (b) G. Chen, F. Song, J. Wang, Z. Yang, S. Sun, J. Fan, X. Qiang, X. Wang, B. Dou and X. Peng, Chem. Commun., 2012, 48, 2949 RSC.
- S. Chen, J. Lu, C. Sun and H. Ma, Analyst, 2010, 135, 577 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details and characterization data. See DOI: 10.1039/c4ra07009g |
| This journal is © The Royal Society of Chemistry 2014 |