Hierarchical PS/PANI nanostructure supported Cu(ii) complexes: facile synthesis and study of catalytic applications in aerobic oxidation†
Abstract
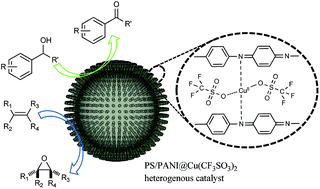
Hierarchical heterogeneous copper catalysts were prepared by immobilization of a homogeneous copper(II) complex on the surface of polystyrene/polyaniline (PS/PANI) microspheres with oriented PANI nanofibers. EDX element maps and XPS spectra indicated that Cu2+ ions strongly coordinated with PANI imine. PS/PANI@Cu(OSO2CF3)2 exhibited excellent catalytic activity for selective aerobic oxidation of alcohols and highly efficient aerobic epoxidation of alkenes under mild conditions. The supported copper(II) catalyst maintained high levels of conversion and selectivity in these reactions after six cycles and showed good stability.

 Please wait while we load your content...
Please wait while we load your content...