A simple and green approach for the synthesis of polyfunctionalized mono- and bis-dihydro-2-oxopyrroles catalyzed by trityl chloride†
Seyed Sajad Sajadikhah*a and
Malek Taher Maghsoodloub
aDepartment of Chemistry, Payame Noor University, Iran. E-mail: sssajadi@pnu.ac.ir
bDepartment of Chemistry, Faculty of Science, University of Sistan and Baluchestan, P. O. Box 98135-674, Zahedan, Iran
First published on 5th September 2014
Abstract
A simple synthesis of ployfunctionalized mono- and bis-dihydro-2-oxopyrrole derivatives is described by one-pot multi-component reaction of amines, dialkyl acetylenedicarboxylates and formaldehyde in the presence of trityl chloride (10 mol%) at room temperature in EtOH. The features of this procedure are mild and green reaction conditions, good to high yields, short reaction times, high atom economy, operational simplicity and no need for column chromatography.
Introduction
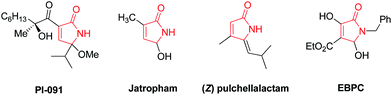
Green chemistry and development of new processes that reduce pollution in chemical synthesis have received considerable attention due to increasing environmental concerns. Among other factors, two major adverse effects on the environment are consumption of energy for heating and cooling of reactions and using volatile organic solvents. The performance of reactions at ambient temperature in green solvents such as water and ethanol may be a viable option.1,2 On the other hand, one active area to access green chemistry is multi-component reactions (MCRs). MCRs have merits over conventional multistage syntheses in several aspects including flexibility, no separation of intermediates and simple purification of products, time and energy saving, and rapid access to complex molecules.3,4 Therefore, discovery and development of new MCRs is highly desirable.Dihydro-2-oxopyrroles (dihydropyrrol-2-ones) display a broad spectrum of biological and pharmacological activity as antitumor and anticancer,5 HIV integrase,6 DNA polymerase inhibitors,7 human cytomegalovirus (HCMV) protease inhibitors,8 and inhibitors of human cytosolic carbonic anhydrase isozymes.9 Dihydro-2-oxopyrrole moiety was also found in various natural bioactive products including pyrrocidine A, talaroconvolutin A, thiomarinol A4, oteromycin, PI-091, EBPC, UCS1025A, Jatropham and (Z) pulchellalactam (Fig. 1).10–16
These heterocycles and derivatives are also active and important reagents as a material for the synthesis of organic complexes.17 As a result, several methods have been developed to synthesize these useful heterocycles.16,18–22 Recently, Zhu et al. have reported an efficient multi-component synthesis of highly functionalized dihydro-2-oxopyrroles via reaction of amines, acetylenic esters and aldehydes in the presence of acetic acid as catalyst. However, the reactions were carried out in the presence of 200 mol% of catalyst at 70 °C and products purified by preparative TLC.23 Next, literature reveals only a few methods to synthesis polyfunctionalized dihydro-2-oxopyrroles via MCRs catalyzed by I2,24 benzoic acid,25 TiO2 nanopowder,26 and Cu(OAc)2·H2O.27 The aforesaid methods have some of the disadvantages such as long reaction times,25 utilization of chlorinated solvent under reflux conditions and need to column chromatography for products purification.26 Therefore, development of an efficient, milder, green and more ecofriendly method for the preparation of these compounds is still in demand.
The utility of organic catalysts in organic synthesis has received a great deal of interest because of their unique properties such as, the possibility to perform reactions for acid-sensitive substrates, performing reactions in milder conditions and selectivity. In the recent years, triarylmethyl chlorides (Ar3CCl) have been used as novel organic catalyst for the synthesis of many organic compounds.28–30 Triarylmethyl chlorides are inexpensive and can be obtained commercially or easily prepared by a known procedure.31
Results and discussion
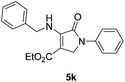
As a part of our continued interest in the development of efficient procedure for the preparation of dihydro-2-oxopyrroles,32–35 herein we report a green, very simple and efficient route for the synthesis of ployfunctionalized dihydro-2-oxopyrroles via one-pot domino four-component reaction of amines, dialkyl acetylenedicarboxylates and formaldehyde. The reaction was carried out in the presence of the catalytic amount of trityl chloride (TrCl) in EtOH at ambient temperature (Scheme 1).Initially, a test reaction using aniline, dimethyl acetylenedicarboxylate (DMAD) and formaldehyde was performed in the absence of catalyst in EtOH at room temperature. The corresponding product 5a was obtained in trace amounts after 24 h. To optimize the reaction conditions, the above reaction was examined under different conditions and the results are presented in Table 1. The best result was obtained in the presence of 10 mol% TrCl in EtOH. As shown in Table 1, higher percentage loading of the catalyst neither increased the yield of product nor decreased the reaction time. Additionally, the effect of different solvents including MeOH, MeCN and THF was also investigated on the yield and time of reaction, which found to be ineffective. A similar result was also obtained in the synthesis of 5k when benzylamine was treated with diethyl acetylenedicarboxylate, formaldehyde and aniline.
| Substrate | Compound | Catalyst (mol%)/solvent | Time (h) | Yieldc (%) |
|---|---|---|---|---|
| a Aniline (1 mmol), DMAD (1 mmol), formaldehyde (1.5 mmol), aniline (1 mmol), r.t.b Benzyl amine (1 mmol), Diethyl acetylenedicarboxylate (1 mmol), formaldehyde (1.5 mmol), aniline (1 mmol), r.t.c Isolated yield. | ||||
 |
 |
—/EtOH | 24 | Trace |
| 5/EtOH | 6 | 60 | ||
| 10/EtOH | 4 | 86 | ||
| 15/EtOH | 4 | 86 | ||
| 20/EtOH | 4 | 85 | ||
| 10/MeOH | 6 | 80 | ||
| 10/MeCN | 9 | 52 | ||
| 10/THF | 9 | 39 | ||
 |
 |
5/EtOH | 7 | 63 |
| 10/EtOH | 4 | 84 | ||
| 15/EtOH | 4 | 82 | ||
| 20/EtOH | 4 | 81 | ||
| 10/MeOH | 8 | 79 | ||
| 10/MeCN | 10 | 51 | ||
| 10/THF | 10 | 40 | ||
Therefore, we investigated several reactions between variety of anilines, dimethyl and/or diethylacetylenedicarboxylate and formaldehyde under optimized reaction conditions. The results are summarized in Table 2. Anilines with substituents Me, OMe, F, Cl, and Br were reacted efficiently to generate the corresponding polyfunctionalized dihydro-2-oxopyrroles 5a–i in good to high yields (Table 2, entries 1–9). Moreover, to evaluate the generality and versatility of this method, the optimized conditions were used for the synthesis of different highly substituted dihydro-2-oxopyrroles 5j–y. As is clear in Table 2, the reactions of aliphatic amines such as benzyl amine, 1-(pyridin-2-yl)methanamine, n-butyl amine and n-propyl amine with dialkyl acetylenedicarboxylates, formaldehyde and aromatic amines were proceeded smoothly to give the expected products in good yields (Table 2, entries 10–25).
| Entry | R′ | R′′ | Ar | Product | Time (h) | Yielda (%) | M.p. (°C) | Lit. m.p. (°C) |
|---|---|---|---|---|---|---|---|---|
| a Isolated yield. | ||||||||
| 1 | Ph | Me | Ph | 5a | 4 | 86 | 153–155 | 155–156 (ref. 24) |
| 2 | Ph | Et | Ph | 5b | 4 | 84 | 137–139 | 138–140 (ref. 23) |
| 3 | 4-F-C6H4 | Me | 4-F-C6H4 | 5c | 4 | 86 | 163–165 | 163–165 (ref. 33) |
| 4 | 4-Cl-C6H4 | Et | 4-Cl-C6H4 | 5d | 4 | 83 | 168–170 | 168–170 (ref. 34) |
| 5 | 4-Br-C6H4 | Me | 4-Br-C6H4 | 5e | 4 | 87 | 175–177 | 179–180 (ref. 24) |
| 6 | 4-Br-C6H4 | Et | 4-Br-C6H4 | 5f | 4 | 84 | 170–172 | 169–171 (ref. 23) |
| 7 | 4-OMe-C6H4 | Et | 4-OMe-C6H4 | 5g | 4 | 74 | 152–154 | 152–154 (ref. 32) |
| 8 | 4-Me-C6H4 | Me | 4-Me-C6H4 | 5h | 3.5 | 86 | 175–177 | 177–178 (ref. 24) |
| 9 | 4-Me-C6H4 | Et | 4-Me-C6H4 | 5i | 4 | 85 | 133–135 | 131–132 (ref. 23) |
| 10 | Ph-CH2 | Me | Ph | 5j | 3 | 89 | 138–140 | 140–141 (ref. 23) |
| 11 | Ph-CH2 | Et | Ph | 5k | 4 | 84 | 127–129 | 130–132 (ref. 23) |
| 12 | Ph-CH2 | Me | 4-Me-C6H4 | 5l | 4 | 84 | 144–146 | 144–146 (ref. 35) |
| 13 | Ph-CH2 | Me | 4-Cl-C6H4 | 5m | 3.5 | 88 | 145–147 | 147–148 (ref. 24) |
| 14 | Ph-CH2 | Me | 4-Br-C6H4 | 5n | 3 | 89 | 117–118 | 120–121 (ref. 24) |
| 15 | Ph-CH2 | Me | 4-F-C6H4 | 5o | 4 | 85 | 166–168 | 166–168 (ref. 32) |
| 16 | C5H4N-2-CH2 | Me | 4-Cl-C6H4 | 5p | 8 | 74 | 156–158 | — |
| 17 | C5H4N-2-CH2 | Me | 4-Me-C6H4 | 5q | 7 | 70 | 106–108 | 106–108 (ref. 34) |
| 18 | n-C4H9 | Me | Ph | 5r | 5.5 | 77 | 62–63 | 60 (ref. 24) |
| 19 | n-C4H9 | Me | 4-Br-C6H4 | 5s | 4 | 82 | 103–105 | 108–109 (ref. 24) |
| 20 | n-C4H9 | Et | 4-Br-C6H4 | 5t | 4 | 85 | 94–96 | 94–96 (ref. 33) |
| 21 | n-C4H9 | Me | 4-F-C6H4 | 5u | 4 | 79 | 81–83 | 81–83 (ref. 35) |
| 22 | n-C4H9 | Me | 3,4-Cl2-C6H4 | 5v | 4 | 80 | 97–99 | 97–99 (ref. 32) |
| 23 | n-C4H9 | Me | 4-Me-C6H4 | 5w | 5 | 83 | 89–91 | — |
| 24 | n-C3H7 | Et | Ph | 5x | 4.5 | 86 | 76–78 | 78–79 (ref. 23) |
| 25 | n-C3H7 | Me | 3,4-Cl2-C6H3 | 5y | 6 | 82 | 125–127 | — |
Interestingly, this approach can be employed for the synthesis of new class of ployfunctionalized bis-dihydro-2-oxopyrroles 7 when ethane-1,2-diamine 6 was used instead of amine 1 (Scheme 2).
The one-pot four-component (pseudo seven-component) reaction of ethane-1,2-diamine 6, dialkyl acetylenedicarboxylate 2, aromatic amine 3 and formaldehyde 4 was carried out cleanly under optimized reaction conditions to generate the corresponding products 7a–g. The results are displayed in Table 3.
| Entry | R′′ | Ar | Product | Time (h) | Yielda (%) | M.p. (°C) | Lit. m.p. (°C) |
|---|---|---|---|---|---|---|---|
| a Isolated yields. | |||||||
| 1 | Et | Ph | 7a | 6 | 79 | 159–161 | — |
| 2 | Me | Ph | 7b | 6 | 81 | 150–152 | 149–151 (ref. 34) |
| 3 | Et | 4-OMe-C6H4 | 7c | 8 | 71 | 222–224 | — |
| 4 | Et | 4-Me-C6H4 | 7d | 6 | 77 | 210–212 | 210–212 (ref. 34) |
| 5 | Me | 4-Cl-C6H4 | 7e | 6 | 82 | 202–204 | — |
| 6 | Et | 3,4-Cl2-C6H3 | 7f | 7 | 80 | 206–208 | 206–208 (ref. 34) |
| 7 | Me | 4-F-C6H4 | 7g | 6 | 83 | 198–200 | 199–201 (ref. 34) |
In general, at the beginning of the reaction, the substrates were completely soluble in reaction medium to form a homogeneous mixture. But, at the end of the reaction, the system became a suspension and finally the product precipitated. The solid precipitate was filtered off and washed with EtOH and purified by recrystallization from EtOH, if necessary. The structures of compounds were fully characterized by IR, 1H and 13C NMR, mass spectra as well as elemental analysis. The structural elucidation of 7a is discussed as an example. The 1H NMR spectrum of 7a showed a triplet at δ 1.27 ppm (J = 6.8 Hz) for methyl protons of ethoxy groups and a multiplet at δ 4.12–4.16 for 4CH2 of ethoxy groups and ethane-1,2-diamine moiety. The methylene protons of dihydro-2-oxopyrrole ring were appeared at δ 4.36 ppm as a singlet. A fairly broad singlet was observed at δ 6.74 ppm for two amine NH groups. The aromatic protons were shown as two triplets and a doublet at δ 7.19 (J = 7.6 Hz), 7.39 (J = 8.0 Hz) and 7.75 ppm (J = 7.6 Hz), respectively. The 1H decoupled 13C NMR spectrum of 7a exhibited 11 distinct signals in agreement with the proposed structure. The mass spectrum of 7a displayed the molecular ion peak (M+) at m/z = 518, which was consistent with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct of ethane-1,2-diamine, diethyl acetylenedicarboxylate, aniline and formaldehyde, respectively. The IR spectrum of compound 7a showed frequencies expected for the NH and carbonyl groups at 3295 cm−1 (NH) and 1698 and 1638 cm−1 (C
2 adduct of ethane-1,2-diamine, diethyl acetylenedicarboxylate, aniline and formaldehyde, respectively. The IR spectrum of compound 7a showed frequencies expected for the NH and carbonyl groups at 3295 cm−1 (NH) and 1698 and 1638 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
A possible reaction mechanism is suggested in Scheme 3. At first, the reaction of amine 1 (or 6) with dialkyl acetylenedicarboxylate 2 lead to intermediate I and condensation between amine 3 and formaldehyde 4 in the presence of TrCl produce imine II. Next, intermediate I undergoes Mannich type reaction with imine II to furnish reactive intermediate III, which converted to intermediate IV by cyclization reaction. Finally, intermediate IV tautomerizes to the corresponding mono- or bis-dihydro-2-oxopyrroles 5 or 7.
Experimental
General
Melting points and IR spectra were taken on an Electrothermal 9100 apparatus and a JASCO FT/IR-460 plus spectrometer, respectively. The 1H and 13C NMR spectra were recorded on a Bruker DRX-400 Avanve instrument with CDCl3 as solvent at 400 and 100 MHz, respectively. Elemental analyses for C, H and N were performed using a Heraeus CHN-O-Rapid analyzer. The mass spectra were recorded on an Agilent Technology (HP) mass spectrometer, operating at an ionization potential of 70 eV. All chemicals were obtained from Merck (Darmastadt, Germany), Acros (Geel, Belgium) and Fluka (Buchs, Switzerland), and use without further purification.General procedure for the synthesis of highly functionalized mono- and bis-dihydro-2-oxopyrroles 5 and 7
For product 5, a mixture of amine 1 (1 mmol) and dialkyl acetylenedicarboxylate 2 (1 mmol) in EtOH (3 mL) was stirred for 20 min. Next, aromatic amine 3 (1 mmol), formaldehyde 4 (37% solution, 1.5 mmol) and TrCl (10 mol%) were added successively. For compound 7, the stoichiometric amounts of amine 6, dialkyl acetylenedicarboxylate 2, amine 3 and formaldehyde 4 are 1, 2, 3 and 3 mmol, respectively. The reaction mixture was stirred at room temperature for appropriate time. The reaction progress was monitored by TLC. After completion, the solid precipitate was filtered off and washed with EtOH (2 mL) and recrystallized from EtOH (if necessary), to give the pure products 5 or 7. The structures of the known products were confirmed by comparison of their melting points and spectral data (IR, 1H and 13C NMR) with literature.23,24,32–35 Physical and chemical data for unknown compounds are presented below.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1628 (C
O), 1628 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (400 MHz, CDCl3): δ = 3.84 (s, 3H, OCH3), 4.45 (s, 2H, CH2–N), 5.25 (d, J = 5.2 Hz, 2H, CH2–NH), 7.23–7.77 (m, 8H, NH and ArH), 8.64 (d, J = 4.4 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ = 47.1, 48.0, 51.2, 120.3, 122.0, 122.4, 129.1, 130.1, 137.2, 137.3, 148.7, 157.1, 164.7, 169.5; MS (EI, 70 eV): m/z (%) = 359 (M2+, 21), 357 (M+, 65), 327 (17), 325 (48), 298 (10), 279 (4), 247 (5), 192 (24) 190 (32), 171 (33), 158 (82), 144 (88), 111 (19), 93 (100), 65 (36); anal. calcd for C18H16ClN3O3: C, 60.42; H, 4.51; N, 11.74. Found: C, 60.71, H, 4.60, N, 11.87.
O); 1H NMR (400 MHz, CDCl3): δ = 3.84 (s, 3H, OCH3), 4.45 (s, 2H, CH2–N), 5.25 (d, J = 5.2 Hz, 2H, CH2–NH), 7.23–7.77 (m, 8H, NH and ArH), 8.64 (d, J = 4.4 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ = 47.1, 48.0, 51.2, 120.3, 122.0, 122.4, 129.1, 130.1, 137.2, 137.3, 148.7, 157.1, 164.7, 169.5; MS (EI, 70 eV): m/z (%) = 359 (M2+, 21), 357 (M+, 65), 327 (17), 325 (48), 298 (10), 279 (4), 247 (5), 192 (24) 190 (32), 171 (33), 158 (82), 144 (88), 111 (19), 93 (100), 65 (36); anal. calcd for C18H16ClN3O3: C, 60.42; H, 4.51; N, 11.74. Found: C, 60.71, H, 4.60, N, 11.87.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1634 (C
O), 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (400 MHz, CDCl3): δ = 0.97 (t, J = 7.2 Hz, 3H, CH3), 1.44 (sextet, J = 7.2 Hz, 2H, CH2), 1.62 (quintet, J = 7.2 Hz, 2H, CH2), 2.36 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 3.89 (t, J = 6.8 Hz, 2H, CH2–NH), 4.39 (s, 2H, CH2–N), 6.76 (br s, 1H, NH), 7.21 (d, J = 8.4 Hz, 2H, ArH), 7.64 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (100 MHz, CDCl3): δ = 13.8, 19.8, 20.8, 33.4, 42.5, 48.0, 50.9, 97.5, 119.4, 129.6, 134.7, 136.3, 164.3, 165.5; MS (EI, 70 eV): m/z, (%) = 302 (M+, 74), 287 (4), 271 (8), 259 (57), 243 (100), 241 (62), 227 (73) 199 (14), 187 (17), 172 (9), 159 (19), 118 (30), 91 (42), 80 (21), 66 (34), 55 (19); anal. calcd for C17H22N2O3: C, 67.53; H, 7.33; N, 9.26. Found: C, 67.42, H, 7.39, N, 9.33.
O); 1H NMR (400 MHz, CDCl3): δ = 0.97 (t, J = 7.2 Hz, 3H, CH3), 1.44 (sextet, J = 7.2 Hz, 2H, CH2), 1.62 (quintet, J = 7.2 Hz, 2H, CH2), 2.36 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 3.89 (t, J = 6.8 Hz, 2H, CH2–NH), 4.39 (s, 2H, CH2–N), 6.76 (br s, 1H, NH), 7.21 (d, J = 8.4 Hz, 2H, ArH), 7.64 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (100 MHz, CDCl3): δ = 13.8, 19.8, 20.8, 33.4, 42.5, 48.0, 50.9, 97.5, 119.4, 129.6, 134.7, 136.3, 164.3, 165.5; MS (EI, 70 eV): m/z, (%) = 302 (M+, 74), 287 (4), 271 (8), 259 (57), 243 (100), 241 (62), 227 (73) 199 (14), 187 (17), 172 (9), 159 (19), 118 (30), 91 (42), 80 (21), 66 (34), 55 (19); anal. calcd for C17H22N2O3: C, 67.53; H, 7.33; N, 9.26. Found: C, 67.42, H, 7.39, N, 9.33.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1643 (C
O), 1643 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (400 MHz, CDCl3): δ = 1.00 (t, J = 7.2 Hz, 3H, CH3), 1.64 (sextet, J = 7.2 Hz, 2H, CH2), 3.81 (s, 3H, OCH3), 3.81–3.83 (m, 2H, CH2–NH), 4.37 (s, 2H, CH2–N), 6.72 (br s, 1H, NH), 7.45 (d, J = 8.8 Hz, 1H, ArH), 7.66 (dd, J = 8.4, 2.4 Hz, 1H, ArH), 8.00 (d, J = 2.4 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ = 11.1, 24.5, 44.4, 47.7, 51.0, 96.3, 117.9, 120.5, 128.1, 130.5, 133.0, 138.2, 164.5, 165.5; MS (EI, 70 eV): m/z, (%) = 344 (M2+, 24), 342 (M+, 34), 315 (26), 313 (45), 295 (15), 285 (47), 283 (100), 241 (10), 226 (5), 187 (10), 174 (15), 172 (18), 145 (13), 112 (16), 80 (23), 66 (33), 53 (16); anal. calcd for C15H16Cl2N2O3: C, 52.49; H, 4.70; N, 8.16. Found: C, 52.76, H, 4.81, N, 8.23.
O); 1H NMR (400 MHz, CDCl3): δ = 1.00 (t, J = 7.2 Hz, 3H, CH3), 1.64 (sextet, J = 7.2 Hz, 2H, CH2), 3.81 (s, 3H, OCH3), 3.81–3.83 (m, 2H, CH2–NH), 4.37 (s, 2H, CH2–N), 6.72 (br s, 1H, NH), 7.45 (d, J = 8.8 Hz, 1H, ArH), 7.66 (dd, J = 8.4, 2.4 Hz, 1H, ArH), 8.00 (d, J = 2.4 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ = 11.1, 24.5, 44.4, 47.7, 51.0, 96.3, 117.9, 120.5, 128.1, 130.5, 133.0, 138.2, 164.5, 165.5; MS (EI, 70 eV): m/z, (%) = 344 (M2+, 24), 342 (M+, 34), 315 (26), 313 (45), 295 (15), 285 (47), 283 (100), 241 (10), 226 (5), 187 (10), 174 (15), 172 (18), 145 (13), 112 (16), 80 (23), 66 (33), 53 (16); anal. calcd for C15H16Cl2N2O3: C, 52.49; H, 4.70; N, 8.16. Found: C, 52.76, H, 4.81, N, 8.23.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1638 (C
O), 1638 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (400 MHz, CDCl3): δ = 1.27 (t, J = 6.8 Hz, 6H, 2OCH2CH3), 4.12–4.16 (m, 8H, 2OCH2CH3 and 2CH2–NH), 4.36 (s, 4H, 2CH2–N), 6.74 (br s, 2H, 2NH), 7.19 (t, J = 7.6 Hz, 2H, ArH), 7.39 (t, J = 8.0 Hz, 4H, ArH), 7.75 (d, J = 7.6 Hz, 4H, ArH); 13C NMR (100 MHz, CDCl3): δ = 14.4, 43.8, 47.9, 59.8, 98.0, 119.3, 124.9, 129.0, 138.7, 164.5, 165.0; MS (EI, 70 eV): m/z, (%) = 518 (M+, 6), 472 (4), 426 (6), 272 (100), 259 (26), 213 (68), 199 (58), 187 (36), 173 (13), 158 (9), 104 (25), 66 (32), 55 (20); anal. calcd for C28H30N4O6: C, 64.85; H, 5.83; N, 10.80. Found: C, 65.04, H, 5.90, N, 10.95.
O); 1H NMR (400 MHz, CDCl3): δ = 1.27 (t, J = 6.8 Hz, 6H, 2OCH2CH3), 4.12–4.16 (m, 8H, 2OCH2CH3 and 2CH2–NH), 4.36 (s, 4H, 2CH2–N), 6.74 (br s, 2H, 2NH), 7.19 (t, J = 7.6 Hz, 2H, ArH), 7.39 (t, J = 8.0 Hz, 4H, ArH), 7.75 (d, J = 7.6 Hz, 4H, ArH); 13C NMR (100 MHz, CDCl3): δ = 14.4, 43.8, 47.9, 59.8, 98.0, 119.3, 124.9, 129.0, 138.7, 164.5, 165.0; MS (EI, 70 eV): m/z, (%) = 518 (M+, 6), 472 (4), 426 (6), 272 (100), 259 (26), 213 (68), 199 (58), 187 (36), 173 (13), 158 (9), 104 (25), 66 (32), 55 (20); anal. calcd for C28H30N4O6: C, 64.85; H, 5.83; N, 10.80. Found: C, 65.04, H, 5.90, N, 10.95.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1634 (C
O), 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (400 MHz, CDCl3): δ = 1.28 (t, J = 7.2 Hz, 6H, 2OCH2CH3), 3.84 (s, 6H, 2OCH3), 4.16–4.19 (m, 8H, 2OCH2CH3 and 2CH2–NH), 4.33 (s, 4H, 2CH2–N), 6.70 (br s, 2H, 2NH), 6.92 (d, J = 9.2 Hz, 4H, ArH), 7.63 (d, J = 9.2 Hz, 4H, ArH); 13C NMR (100 MHz, CDCl3): δ = 14.4, 41.5, 49.0, 55.5, 59.7, 114.2, 121.3, 131.9, 156.9, 164.1, 165.0; MS (EI, 70 eV): m/z, (%) = 578 (M+, 1), 368 (1), 272 (5), 213 (5), 149 (5), 123 (66), 108 (100), 80 (56), 65 (11), 53 (26); anal. calcd for C30H34N4O8: C, 62.27; H, 5.92; N, 9.68. Found: C, 62.59, H, 6.05, N, 9.80.
O); 1H NMR (400 MHz, CDCl3): δ = 1.28 (t, J = 7.2 Hz, 6H, 2OCH2CH3), 3.84 (s, 6H, 2OCH3), 4.16–4.19 (m, 8H, 2OCH2CH3 and 2CH2–NH), 4.33 (s, 4H, 2CH2–N), 6.70 (br s, 2H, 2NH), 6.92 (d, J = 9.2 Hz, 4H, ArH), 7.63 (d, J = 9.2 Hz, 4H, ArH); 13C NMR (100 MHz, CDCl3): δ = 14.4, 41.5, 49.0, 55.5, 59.7, 114.2, 121.3, 131.9, 156.9, 164.1, 165.0; MS (EI, 70 eV): m/z, (%) = 578 (M+, 1), 368 (1), 272 (5), 213 (5), 149 (5), 123 (66), 108 (100), 80 (56), 65 (11), 53 (26); anal. calcd for C30H34N4O8: C, 62.27; H, 5.92; N, 9.68. Found: C, 62.59, H, 6.05, N, 9.80.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1637 (C
O), 1637 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (400 MHz, CDCl3): δ = 3.69 (s, 6H, 2OCH3), 4.14–4.16 (m, 4H, 2CH2–NH), 4.29 (s, 4H, 2CH2–N), 6.75 (br s, 2H, 2NH), 7.34 (d, J = 8.8 Hz, 4H, ArH), 7.69 (d, J = 8.8 Hz, 4H, ArH); 13C NMR (100 MHz, CDCl3): δ = 43.5, 47.9, 51.1, 98.0, 120.2, 129.1, 130.1, 137.2, 164.4, 165.2; MS (EI, 70 eV): m/z, (%) = 560 (M2+, 1), 558 (M+, 2), 441 (10), 336 (34), 304 (21), 292 (35), 280 (34), 266 (38), 247 (60), 221 (100), 138 (30), 111 (35), 91 (77), 69 (48), 57 (82), 55 (60); anal. calcd for C26H24Cl2N4O6: C, 55.82; H, 4.32; N, 10.02. Found: C, 56.10, H, 4.40, N, 9.13.
O); 1H NMR (400 MHz, CDCl3): δ = 3.69 (s, 6H, 2OCH3), 4.14–4.16 (m, 4H, 2CH2–NH), 4.29 (s, 4H, 2CH2–N), 6.75 (br s, 2H, 2NH), 7.34 (d, J = 8.8 Hz, 4H, ArH), 7.69 (d, J = 8.8 Hz, 4H, ArH); 13C NMR (100 MHz, CDCl3): δ = 43.5, 47.9, 51.1, 98.0, 120.2, 129.1, 130.1, 137.2, 164.4, 165.2; MS (EI, 70 eV): m/z, (%) = 560 (M2+, 1), 558 (M+, 2), 441 (10), 336 (34), 304 (21), 292 (35), 280 (34), 266 (38), 247 (60), 221 (100), 138 (30), 111 (35), 91 (77), 69 (48), 57 (82), 55 (60); anal. calcd for C26H24Cl2N4O6: C, 55.82; H, 4.32; N, 10.02. Found: C, 56.10, H, 4.40, N, 9.13.Conclusions
In conclusion, we have developed a highly efficient and green approach for the synthesis of polyfunctionalized mono- or bis-dihydro-2-oxopyrroles via one-pot, four-component reaction of amines, dialkyl acetylenedicarboxylates and formaldehyde using TrCl as a homogeneous catalyst in EtOH at ambient temperature. The presented method offers several advantages including operational simplicity, high atom economy, easy work-up and no need to column chromatography, high yields, mild and environmentally benign reaction conditions.Acknowledgements
Financial support from the Research Council of the Payame Noor University and the University of Sistan and Baluchestan is gratefully acknowledged.Notes and references
- M. Doble and A. Kumar, Green Chemistry and Engineering, Elsevier, 2007 Search PubMed.
- M. G. Dekamin, M. Azimoshan and L. Ramezani, Green Chem., 2013, 15, 811–820 RSC.
- Multicomponent reactions, ed. J. Zhu and H. Bienaymé, Wiley, Weinheim, 2005 Search PubMed.
- A. V. Ivachtchenko, Y. A. Ivanenkov, V. M. Kysil, M. Y. Krasavin and A. P. Ilyin, Russ. Chem. Rev., 2010, 79, 787–817 CrossRef CAS PubMed.
- B. Li, M. P. A. Lyle, G. Chen, J. Li, K. Hu, L. Tang, M. A. Alaoui-Jamali and J. Webster, Bioorg. Med. Chem., 2007, 15, 4601–4608 CrossRef CAS PubMed.
- T. Kawasuji, M. Fuji, T. Yoshinaga, A. Sato, T. Fujiwarab and R. Kiyamaa, Bioorg. Med. Chem., 2007, 15, 5487–5492 CrossRef CAS PubMed.
- Y. Mizushina, S. Kobayashi, K. Kuramochi, S. Nagata, F. Sugawara and K. Sakaguchi, Biochem. Biophys. Res. Commun., 2000, 273, 784–788 CrossRef CAS PubMed.
- A. D. Borthwick, A. J. Crame, P. F. Ertl, A. M. Exall, T. M. Haley, G. J. Hart, A. M. Mason, A. M. K. Pennell, O. M. P. Singh, G. G. Weingarten and J. M. Woolven, J. Med. Chem., 2002, 45, 1–18 CrossRef CAS PubMed.
- C. Alp, D. Ekinci, M. S. Gültekin, M. Şentürk, E. Şahin and Ö. İ. Küfrevioğlu, Bioorg. Med. Chem., 2010, 18, 4468–4474 CrossRef CAS PubMed.
- H. He, H. Y. Yang, R. Bigelis, E. H. Solum, M. Greenstein and G. T. Carter, Tetrahedron Lett., 2002, 43, 1633–1636 CrossRef CAS.
- S. Suzuki, T. Hosoe, K. Nozawa, K.-I. Kawai, T. Yaguchi and S.-I. Udagawa, J. Nat. Prod., 2000, 63, 768–772 CrossRef CAS PubMed.
- H. Shiozawa and S. J. Takahashi, Antibiotics, 1994, 47, 851–853 CrossRef CAS.
- R. Shiraki, A. Sumino, K. Tadano and S. Ogawa, J. Org. Chem., 1996, 61, 2845–2852 CrossRef CAS PubMed.
- R. M. Wiedhopf, E. R. Trumbull and J. R. Cole, J. Pharm. Sci., 1973, 62, 1206–1207 CrossRef CAS.
- W.-R. Li, S. T. Lin, N.-M. Hsu and M.-S. Chern, J. Org. Chem., 2002, 67, 4702–4706 CrossRef CAS PubMed.
- A. Alizadeh, J. Mokhtari and L.-G. Zhu, C. R. Chim., 2013, 16, 863–867 CrossRef CAS PubMed.
- L. N. Aldrich, S. L. Stoops, B. C. Crews, L. J. Marnett and C. W. Lindsley, Bioorg. Med. Chem. Lett., 2010, 20, 5207–5211 CrossRef CAS PubMed.
- I. Loke, N. Park, K. Kempf, C. Jagusch, R. Schobert and S. Laschat, Tetrahedron, 2012, 68, 697–704 CrossRef CAS PubMed.
- F. Palacios, J. Vicario and D. Aparicio, Eur. J. Org. Chem., 2006, 2843–2850 CrossRef CAS.
- M. Lautens, W. Han and J. H.-C. Liu, J. Am. Chem. Soc., 2003, 125, 4028–4029 CrossRef CAS PubMed.
- J. Zhang, P. G. Blazecka and J. G. Davidson, Org. Lett., 2003, 5, 553–556 CrossRef CAS PubMed.
- M. Aginagalde, T. Bello, C. Masdeu, Y. Vara, A. Arrieta and F. P. Cossío, J. Org. Chem., 2010, 75, 7435–7438 CrossRef CAS PubMed.
- Q. Zhu, H. Jiang, J. Li, S. Liu, C. Xia and M. Zhang, J. Comb. Chem., 2009, 11, 685–696 CrossRef CAS PubMed.
- A. T. Khan, A. Ghosh and Md. M. Khan, Tetrahedron Lett., 2012, 53, 2622–2626 CrossRef CAS PubMed.
- H. Gao, J. Sun and C.-G. Yan, Tetrahedron, 2013, 69, 589–594 CrossRef CAS PubMed.
- S. Rana, M. Brown, A. Dutta, A. Bhaumik and C. Mukhopadhyay, Tetrahedron Lett., 2013, 54, 1371–1379 CrossRef CAS PubMed.
- L. Lv, S. Zheng, X. Cai, Z. Chen, Q. Zhu and S. Liu, ACS Comb. Sci., 2013, 15, 183–192 CrossRef CAS PubMed.
- A. Khalafi-Nezhad, A. Parhami, A. Zare, A. R. Moosavi-Zare, A. Hasaninejad and F. Panahi, Synthesis, 2008, 617–621 CrossRef CAS PubMed.
- A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare, A. Zare, A. Parhami and A. Khalafi-Nezhad, Appl. Catal., A, 2010, 386, 179–187 CrossRef CAS PubMed.
- A. Khalafi-Nezhad, A. Parhami, A. Zare, A. Nasrollahi-Shirazi, A. R. Moosavi-Zare and A. Hasaninejad, Can. J. Chem., 2008, 86, 456–461 CrossRef CAS.
- W. E. Bachmann, Org. Synth. Coll. John Wiley & Sons, London, 1955, vol. 3, p. 841 Search PubMed.
- S. S. Sajadikhah, N. Hazeri, M. T. Maghsoodlou, S. M. Habibi-Khorassani, A. Beigbabaei and A. C. Willis, J. Iran. Chem. Soc., 2013, 10, 863–871 CrossRef CAS PubMed.
- S. S. Sajadikhah, N. Hazeri, M. T. Maghsoodlou, S. M. Habibi-Khorassani and K. Khandan-Barani, J. Chem. Res., 2013, 37, 40–42 CrossRef CAS.
- S. S. Sajadikhah and N. Hazeri, Res. Chem. Intermed., 2014, 40, 737–748 CrossRef CAS.
- S. S. Sajadikhah, N. Hazeri, M. T. Maghsoodlou and S. M. Habibi-Khorassani, J. Chin. Chem. Soc., 2013, 60, 1003–1006 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Spectral data, FT-IR, 1H, 13C NMR and mass spectra for the presented compounds. See DOI: 10.1039/c4ra06923d |
| This journal is © The Royal Society of Chemistry 2014 |