Glass-like thermal conductivity in SrTiO3 thermoelectrics induced by A-site vacancies†
Abstract
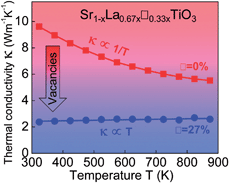
The introduction of A-site vacancies in SrTiO3 results in a glass-like thermal conductivity while Nb substituted samples maintain good electrical conductivity. This unexpected result brings SrTiO3 one step closer to being a high-performing phonon-glass electron-crystal thermoelectric material.

 Please wait while we load your content...
Please wait while we load your content...