Urine metabolomic study of primary dysmenorrhea patients during menstrual period using an ultra performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry (UPLC-Q-TOF-MS)†
Abstract
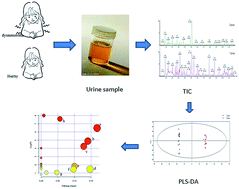
Primary dysmenorrhea (PD) is a common gynecological disease that can seriously affect women's health, lives, and work. Numerous clinical data have shown that the majority of endometriosis and ovarian cancer patients have symptoms of dysmenorrhea. In this study, we established a method based on metabolomic profiling to investigate the differences in small molecule metabolites in urine samples between PD patients and healthy controls. All samples were measured by ultra performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry and analyzed by multivariate statistical methods. Findings show that metabolomics is an effective tool for studying the pathogenesis of PD which could quickly determine the important substances involved in PD, which may be beneficial for the clinical diagnosis and treatment of this disease.

 Please wait while we load your content...
Please wait while we load your content...