Efficient one-pot synthesis of deoxyfructosazine and fructosazine from D-glucosamine hydrochloride using a basic ionic liquid as a dual solvent-catalyst†
Lingyu Jiaab,
Yingxiong Wanga,
Yan Qiaoa,
Yongqin Qia and
Xianglin Hou*a
aState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, 27 South Taoyuan Road, 030001, Taiyuan, PR China. E-mail: houxl@sxicc.ac.cn; Fax: +86 351 4041153; Tel: +86 351 4049501
bUniversity of Chinese Academy of Sciences, 100049, Beijing, PR China
First published on 9th September 2014
Abstract
An efficient one-pot dehydration process for convert D-glucosamine hydrochloride (GlcNH2) into 2-(D-arabino-1′,2′,3′,4′-tetrahydroxybutyl)-5-(D-erythro-2′′,3′′,4′′-trihydroxybutyl)pyrazine (deoxyfructosazine, DOF) and 2,5-bis-(D-arabino-1,2,3,4-tetrahydroxybutyl)pyrazine (fructosazine, FZ) was reported. A task-specific basic ionic liquid, 1-butyl-3-methylimidazolium hydroxide ([BMIM]OH), was employed as an environmentally-friendly solvent and catalyst. The products were qualitatively and quantitatively characterized by MALDI-TOF-MS, 1H NMR and 13C NMR spectroscopy. The influences of GlcNH2 concentrations, reaction temperature, reaction time, additives and co-solvents on the yields of products were studied. The maximum yield of 49% was obtained in the presence of [BMIM]OH and DMSO under optimized conditions (120 °C, 180 min). In addition, a plausible mechanism was proposed. Our project was to develop efficient, atom economical and eco-compatible routes for the synthesis of heterocyclic compounds from marine biomass (or nitrogen-containing biomass). The obtained aromatic heterocyclic compounds showed potential pharmacological action and physiological effects, and they also could be utilized as flavoring agents in the food industry.
1. Introduction
Diminishing reserves of fossil fuel resources, increasing demand for energy and chemicals and growing concerns about environmental issues have led to significant research efforts on the utilization of renewable, bio-sourced feedstocks.1 So far, substantial progress has been made in the production of fuels and value-added platform chemicals, such as 5-hydroxymethylfurfural (5-HMF) and levulinic acid (LA), from lignocellulosic biomass.2,3 However, the investigation and utilization of marine biomass, such as chitin and chitosan, which are the second most abundant non-edible polysaccharides after cellulose has, to our knowledge, not yet been fully explored.4,5 As a readily available, non-toxic, and environmentally benign natural resources, chitin can be sourced on a large scale annually from crustaceans' shells (the industrial waste material of fisheries) and exoskeletons of insects. Under alkaline conditions, chitin can be deacetylated to produce chitosan, which is a linear copolymer of D-glucosamine (GlcNH2) and N-acetyl-D-glucosamine (GlcNAc).6 Compared with the lignocellulosic biomass, the most remarkable potential application of marine biomass is that it can serve as the source of biologically-fixed nitrogen. Commonly, the incorporation of heteroatoms, such as nitrogen, give rise to various unique properties of chemical compounds, which have advanced applications in the industrial, agricultural, medical, and food fields.7–10 The guiding principle of Green Chemistry is to achieve atom economy, therefore, the utilization of these N-containing polysaccharides should be fully realized in order to achieve maximum benefits. As a result, it will not only alleviate environmental pollution, but also produce high value-added chemicals.Deoxyfructosazine (DOF) and fructosazine (FZ) are two non-volatile (polyhydroxyalkyl)pyrazine derivatives with various applications, such as flavoring agents in food industry. DOF has also been found to exhibit potential pharmacological and physiological activities, especially on treatment and prevention of diabetes and resistance of cancers.11,12 Since increasing interests in their high biological and pharmaceutical activities, several synthetic methods related to the Maillard reaction were developed to produce these N-containing heterocyclic compounds. Recently, selective conversion of mono- and polysaccharides, such as glucose or inulin, to DOF in aqueous solutions with additional ammonium salts were developed.13,14 Moreover, GlcNH2, the monomer unit of chitosan, could undergo self-condensation to produce DOF as the sole product catalyzed by phenylboronic acid and sodium hydroxide with relatively high yield.15 In addition, condensation of two moles of GlcNH2 or D-mannosamine in hot methanol could produce FZ.16 However, the distribution of the reaction products could be easily influenced by the additional amino acids, different kinds of ammonium salts and the pH of the reaction mixture. Because of the complexity of the Maillard reaction, species with different molecular weight, such as furfural, volatile and semivolatile pyrazine derivatives, could be observed as by-products in such reactions.13,15,16 Although high product yield and substrate conversion could be achieved for the catalytic Maillard reactions in aqueous solution, conventional bases or organic acids are commonly employed. Consequently, neutralization and salt removal procedures are necessary, which make reaction processes relatively complex and bring undesired side products to the reaction system.17 Furthermore, reactions typically obtained high yields often at the expense of long reaction time and fast selectivity drops.18 So, it is still highly desirable to develop new and efficient catalytic system to prepare these pyrazine derivatives with high yield and selectivity.
Room temperature ionic liquids (RTILs) have attracted growing interests in green synthesis and process due to their unique chemical and physical properties, such as non-volatile, non-flammable, good stability and high solubility for polymeric materials.19,20 For example, Kerton et al. reported that good yield of 3-acetamido-5-acetylfuran could be obtained by the dehydration of GlcNAc in 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) with boric acid as additive.21 Today applications of ionic liquids are far beyond the fields of being just as alternative solvents. Catalysis in ionic liquids is an ongoing and promising area of research. For example, ionic liquids containing imidazolium cations could efficiently promote reactions as catalyst.22 Basic ionic liquids could replace conventional bases and be used in based-catalyzed processes as environmentally-friendly solvents and catalysts with high activity and selectivity.23 For example, 1-butyl-3-methylimidazolium hydroxide, [BMIM]OH, has been applied to catalyze a number of reactions including Michael addition, Markovnikov addition and Knoevenagel condensation.24,25 Alkylated chitosan could be achieved with basic ionic liquid [BMIM]OH as alkaline reagent.26 Although basic ionic liquids have been successfully employed as a catalyst for above mentioned reactions, to our knowledge, there have been no reports of the utilization of [BMIM]OH as an efficient catalyst for the conversion of amino sugars into nitrogen containing compounds.
The work reported herein represents an efficient method for the synthesis of two nonvolatile hydroxyalkyl pyrazine derivatives, DOF and FZ, from the self-condensation of GlcNH2 (Scheme 1). The basic ionic liquid, [BMIM]OH, was employed as both solvent and catalyst. GlcNH2 was served as both substrate and the source of biologically-fixed nitrogen from the viewpoint of atom economy. Moreover, in this reaction process, DOF and FZ were achieved as major components without any conventional by-products produced in previous research work. To the best of our knowledge, no reports have shown the highly selective production of pyrazine derivatives from GlcNH2 under basic imidazolium ionic liquid conditions.
2. Materials and methods
2.1. Materials
Practical grade D-glucosamine hydrochloride (designated as GlcNH2, white crystalline powder) was obtained from Golden-Shell Biochemical Co. Ltd. Deuterium oxide (D2O, 99.9%) and 2,2-dimethyl-2-silapentane-5-sulfonate sodium salt (DSS) were supplied by Qingdao Tenglong Microwave Technology Co. Ltd. 1-Butyl-3-methylimidazolium hydroxide ([BMIM]OH, 12%) was purchased from Shanghai Cheng Jie Chemical Co. Ltd. Dimethyl sulfoxide (DMSO), pyrazine and all other chemicals (analytical grade) were purchased from Sinopharm Chemical Reagent Co. Ltd. All reagents were used without further purification. Deionized water was used in all experiments.2.2. General reaction procedure
In typical experimental procedures, 0.2 g of GlcNH2 was dissolved in 2 g alcoholic solution of 12% alkaline [BMIM]OH with different volume ratio of co-solvent (GlcNH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BMIM]OH, molar ratio 0.6
[BMIM]OH, molar ratio 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Reaction mixture system was loaded in a 10 ml stainless steel vessel with a Teflon lining and sealed by a screw cap following a magnetic bar. The reaction vessel was placed into a pre-heated oil bath and stirred at a speed of 300 rpm at 120 °C for 180 min. A thermostatic oil bath as heating source reaching the desired temperature, zero time was taken. In addition, experiments were conducted at 25, 80, 90, 100, 120, 150 and 180 °C to investigate the effect of reaction temperature. After the reaction, the reaction vessel was taken out from the oil bath and immediately put into ice bath to quench the reaction. The obtained products were diluted by distilled water for further characterization.
1). Reaction mixture system was loaded in a 10 ml stainless steel vessel with a Teflon lining and sealed by a screw cap following a magnetic bar. The reaction vessel was placed into a pre-heated oil bath and stirred at a speed of 300 rpm at 120 °C for 180 min. A thermostatic oil bath as heating source reaching the desired temperature, zero time was taken. In addition, experiments were conducted at 25, 80, 90, 100, 120, 150 and 180 °C to investigate the effect of reaction temperature. After the reaction, the reaction vessel was taken out from the oil bath and immediately put into ice bath to quench the reaction. The obtained products were diluted by distilled water for further characterization.
2.3. Analytic methods
3. Results and discussion
3.1. Characterization of the products
From the viewpoint of atom economy, the catalytic dehydration of non-edible marine biomass to nitrogen containing compounds has become an attractive topic for carbohydrate-based biomass conversion.5,9,21 We aimed to find milder and more efficient methods for the transformation of amino sugars into organic N-containing platform molecules. Herein, we studied the synthesis of pyrazine derivatives, DOF and FZ, through the self-condensation of GlcNH2 catalyzed by a basic ionic liquid [BMIM]OH via an one-pot one-step reaction route.In a typical reaction, 200 mg of GlcNH2 and 2 g IL(OH)/C2H5OH (12%) were mixed and stirred at 120 °C for 180 min (GlcNH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BMIM]OH, molar ratio 0.6
[BMIM]OH, molar ratio 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). A brown mixture reaction system was achieved and the obtained products were identified by 1H NMR, 13C NMR, and MALDI-TOF-MS techniques, which were shown in the ESI.† Fig. S2† showed a typical mass spectrum of products in negative ion modes using of N-(1-naphthyl) ethylenediamine dihydrochloride (NEDC) as a matrix for MALDI MS analysis of products with a time-of-flight (TOF) mass spectrometer.28,29 Besides the peaks originated from matrix, two strong peaks were observed at m/z 339.132 and 355.129, corresponding to the chloride adduct ions of products, (DOF + 35Cl)− and (FZ + 35Cl)−, respectively. The molecular weights were 304 g mol−1 and 320 g mol−1 respectively, which are in agreement with the molecular weight of DOF and FZ.18 Furthermore, 1H NMR and 13C NMR were further applied to confirm the chemical structures of products, including spectra of pure ionic liquid, which were shown in Fig. S3 and S4.† Compounds were found to be disubstituted pyrazine derivatives identical with authentic sample that had been prepared and characterized in our group (Fig. S5†). Remarkably, typical by-products observed in GlcNH2 conversion, such as 5-HMF, LA and low molecule weight volatile pyrazine derivatives were not detected in our experiments.15,18 In previous research, pyrolysis of solid-state GlcNH2 at high temperature (200 °C) or thermal degradation of GlcNH2 in aqueous solution above 100 °C yielded a mixture of products, such as furans, pyrazines and pyridines.30 Severe reaction conditions and unsuitable reaction systems may be responsible for the production of unwanted by-products so that these methods cannot achieve a selective production of desired compounds. In this report, introducing the application of basic ionic liquid as a dual solvent-catalyst, high efficiency of GlcNH2 conversion could be obtained under this relatively milder condition without any traditional side products. Previous research have already focused on the reaction efficiency of basic ionic liquids, for example, researchers investigated to transform carbohydrates into 5-HMF under alkaline ionic liquids as catalysts.31 Furthermore, Michael addition can be easily achieved with high yield and conversion using basic ionic liquid as catalyst and reaction medium.24,25 As far as we aware, there have been no reports of the application of basic ionic liquids as a dual solvent and catalyst for the conversion of marine biomass into N-containing compounds. Herein, the work represented a feasible way to prepare nitrogen containing compounds from amino sugars catalyzed by basic ionic liquid under milder and more efficient conditions.
1). A brown mixture reaction system was achieved and the obtained products were identified by 1H NMR, 13C NMR, and MALDI-TOF-MS techniques, which were shown in the ESI.† Fig. S2† showed a typical mass spectrum of products in negative ion modes using of N-(1-naphthyl) ethylenediamine dihydrochloride (NEDC) as a matrix for MALDI MS analysis of products with a time-of-flight (TOF) mass spectrometer.28,29 Besides the peaks originated from matrix, two strong peaks were observed at m/z 339.132 and 355.129, corresponding to the chloride adduct ions of products, (DOF + 35Cl)− and (FZ + 35Cl)−, respectively. The molecular weights were 304 g mol−1 and 320 g mol−1 respectively, which are in agreement with the molecular weight of DOF and FZ.18 Furthermore, 1H NMR and 13C NMR were further applied to confirm the chemical structures of products, including spectra of pure ionic liquid, which were shown in Fig. S3 and S4.† Compounds were found to be disubstituted pyrazine derivatives identical with authentic sample that had been prepared and characterized in our group (Fig. S5†). Remarkably, typical by-products observed in GlcNH2 conversion, such as 5-HMF, LA and low molecule weight volatile pyrazine derivatives were not detected in our experiments.15,18 In previous research, pyrolysis of solid-state GlcNH2 at high temperature (200 °C) or thermal degradation of GlcNH2 in aqueous solution above 100 °C yielded a mixture of products, such as furans, pyrazines and pyridines.30 Severe reaction conditions and unsuitable reaction systems may be responsible for the production of unwanted by-products so that these methods cannot achieve a selective production of desired compounds. In this report, introducing the application of basic ionic liquid as a dual solvent-catalyst, high efficiency of GlcNH2 conversion could be obtained under this relatively milder condition without any traditional side products. Previous research have already focused on the reaction efficiency of basic ionic liquids, for example, researchers investigated to transform carbohydrates into 5-HMF under alkaline ionic liquids as catalysts.31 Furthermore, Michael addition can be easily achieved with high yield and conversion using basic ionic liquid as catalyst and reaction medium.24,25 As far as we aware, there have been no reports of the application of basic ionic liquids as a dual solvent and catalyst for the conversion of marine biomass into N-containing compounds. Herein, the work represented a feasible way to prepare nitrogen containing compounds from amino sugars catalyzed by basic ionic liquid under milder and more efficient conditions.
3.2. Reaction parameter optimization
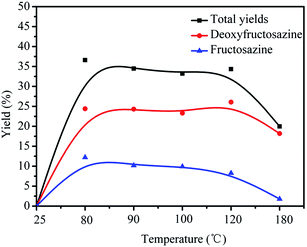 | ||
| Fig. 1 The effect of reaction temperature on the yields of products catalyzed by basic ionic liquid. Reaction conditions: GlcNH2 0.2 g, ionic liquid 2 g, 3 h. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BMIM]OH, molar ratio 0.9
[BMIM]OH, molar ratio 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). It is worth noting that DMSO, in particular, was a much more effective co-solvents. DMSO, as a weak base solvent, could provide good solubility in this condensation reaction of GlcNH2 and also could decrease side reactions in sugars conversion, which were in accordance with previous investigation.33 In addition, it was also found that the signal intensity of chemical shifts of [BMIM]Cl in the 1H NMR gradually decreased with adding DMSO into reaction system, which were shown in Fig. S7.† To some extent, DMSO could decrease the transformation of [BMIM]OH to [BMIM]Cl. These results are in accord with the present scientific judgment of the effect of DMSO on the chemical reactions in general.
1). It is worth noting that DMSO, in particular, was a much more effective co-solvents. DMSO, as a weak base solvent, could provide good solubility in this condensation reaction of GlcNH2 and also could decrease side reactions in sugars conversion, which were in accordance with previous investigation.33 In addition, it was also found that the signal intensity of chemical shifts of [BMIM]Cl in the 1H NMR gradually decreased with adding DMSO into reaction system, which were shown in Fig. S7.† To some extent, DMSO could decrease the transformation of [BMIM]OH to [BMIM]Cl. These results are in accord with the present scientific judgment of the effect of DMSO on the chemical reactions in general.
3.3. Proposed reaction mechanism
GlcNH2, α-amino carbonyl structure from the degradation of chitosan, which is generally considered to be the precursors of pyrazine compounds, can self-react to produce heterocyclic products. The condensation of GlcNH2 is a complex multistep process, and there are several reports on the mechanistic investigation on this industrially significant reaction. All these studies were carried out in aqueous solution with neutral or less acidic conditions. Moreover, all the earlier mechanistic studies were related to the dehydration and keto–enol tautomerization process involved in the reactions.11,13,17,18 However, the mechanism of the condensation reaction in basic ionic liquids has not been fully investigated. Based on previous research, we proposed a plausible reaction mechanism in order to demonstrate the catalysis process with this specific basic ionic liquid. The main reaction pathway from GlcNH2 to pyrazines is a combination of self-condensation and dehydration reactions. [BMIM]OH remained intact in 1H NMR and 13C NMR spectra that obviously indicated that this basic ionic liquid indeed played an important role in the reaction as a catalyst. Pyrazine heterocycles are probably formed by way of cyclization of two acyclic forms molecules through a condensation reaction involving the aldehyde and amino groups. Two possible reaction paths are proposed, which could explain the formation of DOF and FZ respectively. The formation of open chain aldose form of the amino sugars is the first step in the reaction. Mechanistically, the formation of an imine involves two steps in path one, which is used to explain the formation of DOF. First, the nitrogen electron pair acts as a nucleophile, attacking electrophilically activated carbonyl carbon.36 Next step is that the nitrogen is deprotonated, and the electrons from this N–H bond ‘push’ the oxygen off of the carbon, leaving with a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond (an imine) and displaced two water molecules. The end result of this reaction is a compound in which the C
N double bond (an imine) and displaced two water molecules. The end result of this reaction is a compound in which the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond is replaced by a C
O double bond is replaced by a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond. Both the cation and anion of [BMIM]OH were involved in the formation of Schiff base.23,34,37 After that, six-membered heterocycle was formed via an intermolecular nucleophilic cyclization of intermediate, typically called dihydropyrazine.35 The dihydropyrazine intermediate may further eliminate two water molecules to form conjugated polyene system. However, such system is contrary to the rules of Hückel with high energy and cannot exist stably. In fact, closed loop conjugated system tend to be stable with lower energy. Therefore, the dihydropyrazine intermediate only eliminate one water molecule followed by electronic rearrangement process to form a stable six-membered conjugated ring product, called DOF.18
N double bond. Both the cation and anion of [BMIM]OH were involved in the formation of Schiff base.23,34,37 After that, six-membered heterocycle was formed via an intermolecular nucleophilic cyclization of intermediate, typically called dihydropyrazine.35 The dihydropyrazine intermediate may further eliminate two water molecules to form conjugated polyene system. However, such system is contrary to the rules of Hückel with high energy and cannot exist stably. In fact, closed loop conjugated system tend to be stable with lower energy. Therefore, the dihydropyrazine intermediate only eliminate one water molecule followed by electronic rearrangement process to form a stable six-membered conjugated ring product, called DOF.18
Path two is described to explain the formation of FZ. At first, the amine nitrogen acts as a nucleophile, attacking the carbonyl carbon. Contrary to the formation of Schiff base, next step is that the ring carbon is deprotonated to form a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. Six-membered heterocycle, called dihydropyrazine, is also formed.35 Then the N–H bond with weak acid properties is attacked by the hydroxyl anion of [BMIM]OH as base through loss one water molecule. A six-membered conjugated ring is formed through electronic rearrangement. Furthermore, under alkaline conditions, the nitrogen is deprotonated. In the end, another stable six-membered conjugated ring product, called FZ, is formed.18 The conversion of GlcNH2 into DOF and FZ with high atom economy via isomerization and dehydration process in the presence of [BMIM]OH demonstrated that basic ionic liquid could efficient catalyze this reaction. The proposed reaction pathway can be seen in Scheme 2. In addition, conversion of GlcNH2 to pyrazine derivatives under basic conditions often produced side products. The significant side reaction is the condensation of the GlcNH2 to form polymeric caramel colors, which are soluble in aqueous solution. Therefore, undesired side products also produced under basic ionic liquids conditions, which is a major challenge in the synthesis of pyrazines from amino sugars. The structure and amount of water-soluble by-products were under the detection limit of our instrument. However, it is worth noting that insoluble humins, typical side products produced in carbohydrates conversion, were not detected under basic ionic liquids conditions. Moreover, many nitrogenous heterocyclic compounds, such as imidazoles, pyridines, and pyrroles, which have been found in sugar-amine browning reaction, were also not detected in our reaction system.
C double bond. Six-membered heterocycle, called dihydropyrazine, is also formed.35 Then the N–H bond with weak acid properties is attacked by the hydroxyl anion of [BMIM]OH as base through loss one water molecule. A six-membered conjugated ring is formed through electronic rearrangement. Furthermore, under alkaline conditions, the nitrogen is deprotonated. In the end, another stable six-membered conjugated ring product, called FZ, is formed.18 The conversion of GlcNH2 into DOF and FZ with high atom economy via isomerization and dehydration process in the presence of [BMIM]OH demonstrated that basic ionic liquid could efficient catalyze this reaction. The proposed reaction pathway can be seen in Scheme 2. In addition, conversion of GlcNH2 to pyrazine derivatives under basic conditions often produced side products. The significant side reaction is the condensation of the GlcNH2 to form polymeric caramel colors, which are soluble in aqueous solution. Therefore, undesired side products also produced under basic ionic liquids conditions, which is a major challenge in the synthesis of pyrazines from amino sugars. The structure and amount of water-soluble by-products were under the detection limit of our instrument. However, it is worth noting that insoluble humins, typical side products produced in carbohydrates conversion, were not detected under basic ionic liquids conditions. Moreover, many nitrogenous heterocyclic compounds, such as imidazoles, pyridines, and pyrroles, which have been found in sugar-amine browning reaction, were also not detected in our reaction system.
 | ||
| Scheme 2 Proposed reaction pathway for the formation of deoxyfructosazine and fructosazine from GlcNH2. | ||
4. Conclusions
Direct conversion of GlcNH2 into nitrogen containing compounds (DOF and FZ) under basic imidazolium based ionic liquid in good yield was systematically investigated for the first time. Enhanced reaction rate and improved selectivity were achieved using [BMIM]OH as a dual solvent and catalyst. A maximum yield of 49% was obtained with catalytic amount of ionic liquid. This investigation contrasted with recently published work from our group where 5-HMF was obtained as the primary product through conversion of marine biomass in concentrated ZnCl2 aqueous solution. [BMIM]OH and DMSO were identified as the effective catalyst and the best co-solvent respectively. A possible mechanism was proposed. Nitrogen and carbon atom efficiency were quite good up to 100% from the viewpoint of atom economy.Acknowledgements
This work was financially supported by the Major State Basic Research Development Program of China (973 Program) (no. 2012CB215305), the Natural Science Foundation of China (no. 21106172) and Science Foundation of Shanxi Province (2012021009-2). Special thanks to Prof. Zongxiu Nie (Beijing National Laboratory for Molecular Sciences, Key Laboratory of Analytical Chemistry for Living Biosystems, Institute of Chemistry, Chinese Academy of Sciences) for Mass Spectrometry analysis.References
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164–7183 CrossRef CAS PubMed.
- T. Deng, X. Cui, Y. Qi, Y. Wang, X. Hou and Y. Zhu, Chem. Commun., 2012, 48, 5494–5496 RSC.
- C. K. S. Pillai, W. Paul and C. P. Sharma, Prog. Polym. Sci., 2009, 34, 641–678 CrossRef CAS PubMed.
- Y. Wang, C. M. Pedersen, T. Deng, Y. Qiao and X. Hou, Bioresour. Technol., 2013, 143, 384–390 CrossRef CAS PubMed.
- K. W. Omari, J. E. Besaw and F. M. Kerton, Green Chem., 2012, 14, 1480–1487 RSC.
- X. Chen, S. L. Chew, F. M. Kerton and N. Yan, Green Chem., 2014, 16, 2204–2212 RSC.
- Y. Ohmi, S. Nishimura and K. Ebitani, ChemSusChem, 2013, 6, 2259–2262 CrossRef CAS PubMed.
- K. W. Omari, L. Dodot and F. M. Kerton, ChemSusChem, 2012, 5, 1767–1772 CrossRef CAS PubMed.
- M. Osada, K. Kikuta, K. Yoshida, K. Totani, M. Ogata and T. Usui, Green Chem., 2013, 15, 2960–2966 RSC.
- Q. Li, A. Yan, Y. Ye and Q. Xing, Acta Sci. Nat. Univ. Pekin., 2001, 37, 286–288 CAS.
- A. Zhu, J. B. Huang, A. Clark, R. Romero and H. R. Petty, Carbohydr. Res., 2007, 342, 2745–2749 CrossRef CAS PubMed.
- K. Agyei-Aye, M. X. Chian, J. H. Lauterbach and S. C. Moldoveanu, Carbohydr. Res., 2002, 337, 2273–2277 CrossRef CAS.
- S. Wu, H. Fan, Q. Zhang, Y. Cheng, Q. Wang, G. Yang and B. Han, Clean: Soil, Air, Water, 2011, 39, 572–576 CrossRef CAS.
- J. Rohovec, J. Kotek, J. A. Peters and T. Maschmeyer, Eur. J. Org. Chem., 2001, 2001, 3899–3901 CrossRef.
- S. Fujii, R. Kikuchi and H. Kushida, J. Org. Chem., 1966, 31, 2239–2241 CrossRef CAS.
- H. Tsuchida, M. Komoto, H. Kato and M. Fujimaki, Agric. Biol. Chem., 1973, 37, 2571–2578 CrossRef CAS.
- K. Sumoto, M. Irie, N. Mibu, S. Miyano, Y. Nakashima, K. Watanabe and T. Yamaguchi, Chem. Pharm. Bull., 1991, 39, 792–794 CrossRef CAS.
- H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1–56 CrossRef CAS PubMed.
- P. Zhang, Y. Gong, Y. Lv, Y. Guo, Y. Wang, C. Wang and H. Li, Chem. Commun., 2012, 48, 2334–2336 RSC.
- M. W. Drover, K. W. Omari, J. N. Murphy and F. M. Kerton, RSC Adv., 2012, 2, 4642–4644 RSC.
- T. Jiang, X. Ma, Y. Zhou, S. Liang, J. Zhang and B. Han, Green Chem., 2008, 10, 465–469 RSC.
- A. R. Hajipour and F. Rafiee, J. Iran. Chem. Soc., 2009, 6, 647–678 CrossRef CAS.
- B. C. Ranu and S. Banerjee, Org. Lett., 2005, 7, 3049–3052 CrossRef CAS PubMed.
- B. C. Ranu, S. Banerjee and R. Jana, Tetrahedron, 2007, 63, 776–782 CrossRef CAS PubMed.
- L. Pei, Z. Cai, S. Shang and Z. Song, J. Appl. Polym. Sci., 2014, 131, 40052–40058 CrossRef.
- T. Rundlöf, M. Mathiasson, S. Bekiroglu, B. Hakkarainen, T. Bowden and T. Arvidsson, J. Pharm. Biomed. Anal., 2010, 52, 645–651 CrossRef PubMed.
- R. Chen, W. Xu, C. Xiong, X. Zhou, S. Xiong, Z. Nie, L. Mao, Y. Chen and H. Chang, Anal. Chem., 2012, 84, 465–469 CrossRef CAS PubMed.
- S. Chen, H. Zheng, J. Wang, J. Hou, Q. He, H. Liu, C. Xiong, X. Kong and Z. Nie, Anal. Chem., 2013, 85, 6646–6652 CrossRef CAS PubMed.
- J. Chen, M. Wang and C. T. Ho, J. Agric. Food Chem., 1998, 46, 1971–1974 CrossRef CAS.
- Y. Qu, Y. Song, C. Huang, J. Zhang and B. Chen, Ind. Eng. Chem. Res., 2012, 51, 13008–13013 CrossRef CAS.
- Y. Peng, G. Li, J. Li and S. Yu, Tetrahedron Lett., 2009, 50, 4286–4288 CrossRef CAS PubMed.
- H. Wang, Q. Kong, Y. Wang, T. Deng, C. Chen, X. Hou and Y. Zhu, ChemCatChem., 2014, 6, 728–732 CrossRef CAS.
- H. Zang, M. Wang, B. Cheng and J. Song, Ultrason. Sonochem., 2009, 16, 301–303 CrossRef CAS PubMed.
- N. Kashige, T. Yamaguchi, N. Mishiro, H. Hanazono, F. Miake and K. Watanabe, Biol. Pharm. Bull., 1995, 18, 653–658 CAS.
- V. Y. Kukushkin and A. J. L. Pombeiro, Coord. Chem. Rev., 1999, 181, 147–175 CrossRef CAS.
- E. H. Cordes and W. P. Jencks, J. Am. Chem. Soc., 1962, 84, 832–837 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06832g |
| This journal is © The Royal Society of Chemistry 2014 |






