Metal-free syntheses of oxindole derivatives via a benzoylation/substitution/desulfonylation/cyclization cascade†
Ben Niua,
Ping Xieb,
Wannian Zhaoa,
Yang Zhoua,
Zhaogang Biana,
Charles U. Pittman Jr.c and
Aihua Zhou*a
aPharmacy School, Jiangsu University, Xuefu Road 301, Zhenjiang City, Jiangsu 212013, China. E-mail: ahz@ujs.edu.cn; Fax: +86-511-85038451 ext. 806; Tel: +86-511-8503-8895 ext. 812
bScientific Information Research Institute, Jiangsu University (Library), China
cDepartment of Chemistry, Mississippi State University, Mississippi State, MS 39762, USA
First published on 8th September 2014
Abstract
A benzoylation/substitution/desulfonylation/cyclization cascade reaction giving oxindole derivatives was discovered. The reaction used aromatic aldehydes and N-alkyl-N-(phenylsulfonyl)methacrylamides as starting materials, and proceeded under mild conditions without using toxic metal catalysts. 3-Methyl-3-aroyloxindole derivatives were formed in good yields.
Oxindole derivatives are important molecules found in a wide range of natural products. They are highly valuable molecules in drug discovery due to their variety of bioactivities.1 Many oxindole derivatives have been synthesized and screened, and different biological activities have been reported.2 Fig. 1 shows four representative bioactive oxindole derivatives.3 These include convolutamydine A, a natural product with potent activity against leukemia cells.3a Also, selective 5-HT7 receptor antagonists,3b an antitumor agent, and selective inhibitors of the plasmodial CDKs are included.3c,d Thus, quick syntheses of oxindole derivatives are highly valued in high-throughput screening. Such cascade reactions are greatly desired.
Traditional synthetic methods4 for generating oxindole derivatives have been supplemented recently by some powerful cascade methods using transitional metal catalysts to carry out oxidative cross couplings of activated alkenes.5 These methods have attracted a lot of attention. N-Alkyl-N-(aryl)methacrylamides were normally used as core reactants. By carefully designing their substrate structures, different oxindole derivatives were produced in one-pot cascade sequences under mild conditions. These reactions are atom-economic, highly efficient and environmentally friendly.6 More recently, another cascade reaction that produces 3-methyl-3-aroyloxindole derivatives has quickly drawn attention.7 Several functionalizations using N-alkyl-N-(phenylsulfonyl)-methacrylamide as the core reactants have been reported.7
In this paper, a novel cascade reaction involving benzoylation/substitution/desulfonylation/cyclization to produce 3-methyl-3-aroyloxindole derivatives is reported. This process never involves a metal catalyst.
In order to find suitable reaction conditions for this cascade, metal catalysts and radical initiators were initially screened based on previously reported results.8 N-Methyl-N-(phenylsulfonyl)methacrylamide and benzaldehyde were used as representative reactants (Table 1) for screening. In entry 1, CuCl2 (10 mol%) was used as a catalyst in the presence of TBHP (tert-butyl hydroperoxide, 70% in water, 2.5 equiv.). Excess benzaldehyde (5 equiv.) was used to promote conversion, giving 3a in a yield of 35%. Using TBHP as the initiator gave about 40% of 3a. In entry 3, one equivalent of NaHCO3 was also added and 3a was produced in a good yield of 70%. NaHCO3 can increase the yield greatly; because it may consume the SO2 released in the reaction. When TBHP was replaced by DTBP (di-tert-butyl peroxide) in the presence of NaHCO3, 3a was produced in 56% yield. When PhI(OAc)2 and NaHCO3 were both used, none or only traces of desired 3a was detected. Using solvents DCE, toluene (entries 6, 7) afforded none or only traces of 3a, while acetonitrile and EtOAc (entry 8, 9) afforded 20% and 53% of expected product 3a, respectively. Using H2O2 (10 mol%) in the presence of NaHCO3 gave only traces of 3a.
| Entry | Cat. (mol%) | Reagent | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (5 equiv.), N-methyl-N-(phenylsulfonyl)methacrylamide (1 equiv.), aqueous TBHP (tert-butyl hydroperoxide, 70 wt% in water, 2.5 equiv.), H2O2 (30 wt% in water, 2.5 equiv.), DTBP (tert-butyl peroxide, 2.5 equiv.), CuCl2 (10 mol%, for entry 1); NaHCO3 (1 equiv.), reaction time 18 h.b Yield is based on reactant 2a. | ||||
| 1 | CuCl2 | TBHP, NaHCO3 | — | 35 |
| 2 | TBHP | — | 40 | |
| 3 | TBHP, NaHCO3 | — | 70 | |
| 4 | DTBP, NaHCO3 | — | 56 | |
| 5 | PhI(OAc)2, NaHCO3 | — | Trace | |
| 6 | TBHP, NaHCO3 | DCE | Trace | |
| 7 | TBHP, NaHCO3 | Toluene | Trace | |
| 8 | TBHP, NaHCO3 | CH3CN | 20 | |
| 9 | TBHP, NaHCO3 | EtOAc | 53 | |
| 10 | H2O2, NaHCO3 | — | Trace | |
Based on these screening results, the optimized reaction conditions employed were: aldehyde (5 equiv.), TBHP (2.5 equiv.), 90 °C, NaHCO3 (2 equiv.), 18 h. Under these conditions, fourteen reactions with different substituents were thoroughly studied (Table 2). All these cascade reactions gave 3-methyl-3-aroyloxindole derivatives 3a–m in good yields except entry 14 using the electron-withdrawing 3-nitrobenzaldehyde as a starting material. This fact indicates that the nitro function may influence radical reaction process, since it didn't give the expected product.
| Entry | R | R1 | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: aldehydes (5 equiv.), N-alkyl-N-(phenylsulfonyl)methacrylamides (1 equiv.), aqueous TBHP (tert-butyl hydroperoxide, 70 wt% in water, 2.5 equiv.), NaHCO3 (1 equiv.), reaction time 18 h.b Yield calculation is based on reactant 2. | ||||
| 1 | H | Me | 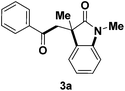 |
70 |
| 2 | 4-MeO | Me |  |
77 |
| 3 | 4-Me | Me |  |
75 |
| 4 | 4-t-Bu | Me |  |
81 |
| 5 | 4-MeO | Et |  |
68 |
| 6 | 4-Me | Et |  |
70 |
| 7 | H | Et |  |
72 |
| 8 | 4-t-Bu | Et |  |
85 |
| 9 | 4-MeO | i-Pr |  |
76 |
| 10 | H | i-Pr |  |
74 |
| 11 | 4-CH3 | i-Pr |  |
73 |
| 12 | 4-Br | i-Pr |  |
70 |
| 13 | 2-F | i-Pr |  |
60 |
| 14 | 3-NO2 | i-Pr |  |
Trace |
Based on previous reports7 and our mass spectrometry analysis results, it is obvious that the SO2 functions were lost during the reaction. No sultams was in the products. To further confirm the product structures; a comparison experiment was conducted (Scheme 1). N-Methyl-N-phenylmethacrylamide was used as a representative starting material to compare with the reaction of 2a. The 1H and 13C-NMR of the products from each reaction confirmed that 3a was produced in both reactions. Obviously, SO2 is lost in this cascade sequence from 2a. This conclusion is further supported by the reactions in ref. 7. Based on the above, a reaction mechanism is proposed in Scheme 2. When heated, TBHP gives a tBuO˙ and an ˙OH radical, which abstract a hydrogen atom from the aryl aldehyde 1 to generate the aroyl radical. This aroyl radical adds to the double bond of N-alkyl-N-(phenylsulfonyl)acrylamide to give delocalized radical intermediate 5, which undergoes intramolecular radical substitution at the aromatic ring with loss of SO2. This forms radical 6. The addition of resultant radical 6 to the aromatic ring generates radical intermediate 7, which loses a hydrogen atom to give ketone oxindole derivatives 3 in good yields.
In summary, we have developed a novel, metal-free cascade reaction involving sequential benzoylation/substitution/desulfonylation/cyclization steps to give 3-methyl-3-aroyloxindole derivatives. The reaction used aromatic aldehydes and N-alkyl-N-(phenylsulfonyl)methacrylamides as starting materials and proceeded under mild and environmentally friendly conditions to give good yields. This result enriches current methods of generating oxindole derivatives. All of these 3-methyl-3-aroyloxindole derivatives will be screened soon for biological activities.
Acknowledgements
We gratefully acknowledge Jiangsu University for financial support (1281290006).Notes and references
- (a) G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104 CrossRef CAS PubMed; (b) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464 RSC; (c) S. R. Chemler, Org. Biomol. Chem., 2009, 7, 3009 RSC; (d) B.-J. Li and Z.-J. Shi, Chem. Soc. Rev., 2012, 41, 5588 RSC; (e) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (f) J. Wencel-Delord, T. Dröge, F. Liuand and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC; (g) J. A. Ashenhurst, Chem. Soc. Rev., 2010, 39, 540 RSC; (h) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (i) D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef PubMed; (j) F. Pan, W. Yu, Z. Qi, C. Qiao and X. Wang, Synthesis, 2014, 09, 1143 Search PubMed; (k) H. Chen, J. Bai, Z. Fang, S. Yu, S. Ma, S. Xu, Y. Li, J. Qu, J. Ren, L. Li, Y. Si and X. Chen, J. Nat. Prod., 2011, 74, 2438 CrossRef CAS PubMed; (l) H. Lin and S. Danishefsky, Angew. Chem., Int. Ed., 2003, 42, 36 CrossRef CAS PubMed; (m) Y.-J. Xie, J. Sun and C.-G. Yan, ACS Comb. Sci., 2014, 16, 271 CrossRef CAS PubMed.
- (a) A. Millemaggi and R. J. Taylor, Eur. J. Org. Chem., 2010, 24, 4527 CrossRef PubMed; (b) E. J. Hennessy and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 12084 CrossRef CAS PubMed; (c) A. Beyer, J. Buendia and C. Bolm, Org. Lett., 2012, 14, 3948 CrossRef CAS PubMed; (d) S. Ueda, T. Okada and H. Nagasawa, Chem. Commun., 2010, 46, 2462 RSC; (e) M. G. LaPorte, S. Tsegay, K. Hong, C. Lu, C. Fang, L. Wang, X. Xie and P. E. Floreancig, ACS Comb. Sci., 2013, 15, 344 CrossRef CAS PubMed; (f) J. P. MacDonald, J. J. Badillo, G. E. Arevalo, A. Silva-García and K. A. Franz, ACS Comb. Sci., 2012, 14, 285 CrossRef CAS PubMed.
- (a) Y. Kamano, H. P. Zhang, Y. Ichihara, H. Kizu, K. Komiyama and G. R. Pettit, Tetrahedron Lett., 1995, 36, 2783 CrossRef CAS; (b) B. Volk, J. Barkoczy, E. Hegedus, S. Udvari, I. Gacsalyi, T. Mezei, K. Pallagi, H. Kompagne, G. Levay, A. Egyed, L. G. Harsing, M. Spedding and G. Simig, J. Med. Chem., 2008, 51, 2522 CrossRef CAS PubMed; (c) M. K. Christensen, K. D. Erichsen, C. Hansen, J. Tjornelund, S. J. Nielsen, K. Frydenvang, T. N. Johansen, B. Nielsen, M. Sehested, P. B. Jensen, M. Ikaunieks, A. Zaichenko, E. Loza, I. Kalvinsh and F. Bjorkling, J. Med. Chem., 2010, 53, 7140 CrossRef CAS PubMed; (d) C. L. Woodard, Z. Li, A. K. Kathcart, J. Terrell, L. Gerena, M. Sanchez, D. E. Kyle, A. K. Bhattacharjee, D. A. Nichols, W. Ellis and S. T. Prigge, J. Med. Chem., 2003, 46, 3877 CrossRef CAS PubMed.
- (a) P. G. Gassman and T. J. Van Bergen, J. Am. Chem. Soc., 1973, 95, 2718 CrossRef CAS; (b) A. Pinto, L. Neuville, P. Retailleau and J. Zhu, Org. Lett., 2006, 8, 4927 CrossRef CAS PubMed; (c) R. Grigg and V. Sridharan, J. Organomet. Chem., 1999, 576, 65 CrossRef CAS; (d) B. M. Trost and M. K. Brennan, Synthesis, 2009, 18, 3003 CrossRef; (e) F. Zhou, Y. Liu and J. Zhou, Adv. Synth. Catal., 2010, 9, 1381 CrossRef PubMed; (f) T. Piou, L. Neuville and J. Zhu, Angew. Chem., Int. Ed., 2012, 46, 11729 CrossRef PubMed; (g) L. Zou, X. Bao, Y. Ma, Y. Song, J. Qu and B. Wang, Chem. Commun., 2014, 50, 5760 RSC.
- (a) W. Wei, M. Zhou, J. Fan, W. Liu, R. Song, Y. Liu, M. Hu, P. Xie and J. Li, Angew. Chem., Int. Ed., 2013, 52, 3638 CrossRef CAS PubMed; (b) S. Zhou, L. Guo, H. Wang and X. Duan, Chem.–Eur. J., 2013, 19, 12970 CrossRef CAS PubMed; (c) J. Liu, S. Zhuang, Q. Gui, X. Chen, Z. Yang and Z. Tan, Eur. J. Org. Chem., 2014, 15, 3196 CrossRef PubMed; (d) M. Zhou, C. Wang, R. Song, Y. Liu, W. Wei and J. Li, Chem. Commun., 2013, 49, 10817 RSC; (e) Y. Li, Y. Shen, K. Chang and S. Yang, Tetrahedron Lett., 2014, 55, 2119 CrossRef CAS PubMed; (f) X. Li, X. Xu, P. Hu, X. Xiao and C. Zhou, J. Org. Chem., 2013, 78, 7343 CrossRef CAS PubMed; (g) Z. Li, Y. Zhang, L. Zhang and Z. Liu, Org. Lett., 2014, 16, 382 CrossRef CAS PubMed; (h) F. Yin and X. Wang, Org. Lett., 2014, 16, 1128 CrossRef CAS PubMed.
- (a) Y. Li, X. Wei, X. Li and S. Yang, Chem. Commun., 2013, 49, 11701 RSC; (b) H. Wang, L. Guo and X. Duan, Org. Lett., 2013, 15, 5254 CrossRef CAS PubMed; (c) W. Wei, J. Wen, D. Yang, J. Du, J. You and H. Wang, Green Chem., 2014, 16, 2988 RSC; (d) Y. Li, Y. Shen, K. Chang and S. Yang, Tetrahedron Lett., 2014, 70, 1991 CrossRef CAS PubMed; (e) T. Shen, Y. Yuan and N. Jiao, Chem. Commun., 2014, 50, 554 RSC.
- (a) W. Kong, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2014, 53, 5078 CAS; (b) W. Kong, M. Casimiro, N. Fuentes, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2013, 52, 13086 CrossRef CAS PubMed; (c) W. Kong, M. Casimiro, E. Merino and C. Nevado, J. Am. Chem. Soc., 2013, 135, 14480 CrossRef CAS PubMed; (d) L. Li, M. Deng, S. Zheng, Y. Xiong, B. Tan and X. Liu, Org. Lett., 2014, 16, 504 CrossRef CAS PubMed.
- (a) W. Gong, L. Xu, T. Ji, P. Xie, X. Qi, C. U. Pittman, Jr and A. Zhou, RSC Adv., 2014, 4, 6854 RSC; (b) M. Zhou, R. Song, X. Ouyang, Y. Liu, W. Wei, G. Deng and J. Li, Chem. Sci., 2013, 4, 2690 RSC; (c) F. Jia, K. Liu, H. Xi, S. Lu and Z. Li, Tetrahedron Lett., 2014, 54, 6337 CrossRef PubMed; (d) G. Packer, K. Leper, J. Kankanala and V. Sridharan, RSC Adv., 2014, 4, 3457 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06810f |
| This journal is © The Royal Society of Chemistry 2014 |





