Homogeneous decoration of zeolitic imidazolate framework-8 (ZIF-8) with core–shell structures on carbon nanotubes†
Abstract
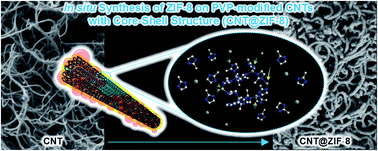
Considerable attention has focused on the combination of carbon nanotubes (CNTs) and metal–organic frameworks (MOFs) since both nanomaterials have outstanding properties. We describe a method for the homogeneous decoration of a MOF (ZIF-8 was chosen) onto the surfaces of CNTs dispersed by polyvinylpyrrolidones (PVPs) in methanol, which was revealed by a scanning electron microscopic study. The homogeneous coating of the MOF on the CNTs, and nanostructures of the CNT-MOF were controlled by simply changing the concentrations of the MOFs. Furthermore, this method was also applicable to graphene and graphene oxide (GO). CO2 uptakes of the CNT-MOF and graphene-MOF were significantly improved as compared to the nonhomogeneous composites synthesized without the PVP functionalization, and a good reproducibility of the CO2 adsorption was confirmed by the cycling test.

 Please wait while we load your content...
Please wait while we load your content...