One-pot synthesis of functionalized β-amino sulfides/β-amino selenides via ring opening of cyclic sulfamidates†
Abstract
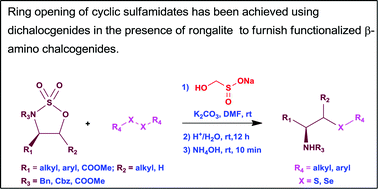
A number of functionalized β-amino and γ-amino sulfides and selenides have been synthesized involving a one-pot process of ring opening of cyclic sulfamidates with ‘in situ’ generated thiolate and selenoate species from diaryl disulfides and diphenyl diselenide using rongalite. A mild and efficient method has been developed for the synthesis of cysteines from serine.

 Please wait while we load your content...
Please wait while we load your content...