Recent development of metal hydroxides as electrode material of electrochemical capacitors
J. P. Cheng
*,
J. Zhang
and
F. Liu
*
Department of Materials Science and Engineering, State Key Laboratory of Silicon Materials, Key Laboratory of Advanced Materials and Applications for Batteries of Zhejiang Province, Zhejiang University, Hangzhou 310027, P.R. China. E-mail: chengjp@zju.edu.cn; liufu@zju.edu.cn; Fax: +86-571-87951411; Tel: +86-571-87951411
First published on 5th August 2014
Abstract
Electrochemical capacitors, also known as supercapacitors, are energy storage devices, characterized by rapid rates of charging and discharging and high power density. In recent years, electrochemical capacitors have attracted significant attention as ‘bridges’ for the power/energy gap between traditional capacitors and batteries/fuel cells. The integrated performance of an electrochemical capacitor is essentially determined by its electrode materials. This is a review of electrode materials for electrochemical capacitors, chiefly concerning transition metal hydroxides. In this work, we focused particularly on recently published reports using cheap metal hydroxides as electrode materials for electrochemical capacitors, based on classification of metal hydroxide by composition and microstructure. Some important experimental data on this issue are indicated and summarized. Furthermore, a brief discussion of future development, challenges, and opportunities in this area is also provided.
1. Introduction
Energy storage is critical for efficient, clean, and versatile use of energy, and is also key in energy harvesting for use as renewable energy. Batteries keep our electronic devices working for a long time because of their high energy densities. For rapid power delivery and recharging, electrochemical capacitors (ECs) are used. Electrochemical capacitors, also called supercapacitors, which can operate at a high charge/discharge rate over a large number of cycles, are one kind of important device for energy storage.1 ECs can store electrochemical energy by either ion adsorption as an electrochemical double layer capacitor (EDLC) or fast surface redox reactions like batteries.2 The performance of ECs is essentially determined by their electrode materials. The most widely known electrode materials for redox actions of ECs are RuO2 and MnO2. Recently, this list has been expanded to include other metal oxides, as well as hydroxides, nitrides, and carbides. Thus, transition metal oxides and hydroxides such as MnO2, Fe3O4, NiCo2O4, V2O5, Ni(OH)2, and Co(OH)2, have been extensively studied as the electrode materials of supercapacitors, enabling fast reversible redox reactions on their surfaces.3 The charge storage mechanism of ECs is generally determined by EDLCs and pseudocapacitors. Although EDLCs based on carbon electrode materials have good stability, their charge mechanism limits specific capacitances at a range of low values. Thus, ECs based on pseudocapacitors usually exhibit much higher specific capacitances than EDLCs. Among the candidate electrode materials for pseudocapacitors, oxides and hydroxides of transition metals present high specific capacitance because of their rich redox properties involving multiple oxidation states.In recent years, there has been great progress in the theoretical and practical research and development of ECs, as evinced by a large number of published research papers. Low-cost and environmentally friendly ECs with capability for storing large amounts of electrical energy have potential applications in electric vehicles, energy storage for renewable energy production, portable electronics, and large industry equipment. Thus, an elegant way of achieving high energy density is to design hybrid systems using earth abundant metal oxides or hydroxides as the electrode materials of ECs. Because there have been many review articles based on the advances of metal oxide electrode materials for ECs,4–13 we will focus on the development of metal hydroxides such as cobalt hydroxide and nickel hydroxide.
In this review, we survey recent reports of earth abundant metal hydroxides as electrode materials of ECs, typically from 2009 to now. In an attempt to review transition metal hydroxide materials for ECs, we discuss their preparation and modification, followed by classification of metal hydroxides by composition and microstructure. They are grouped into several sections including single-, binary metal hydroxides, hydrotalcite-like compounds, hierarchical hybrids of metal hydroxide and metal oxide, and asymmetric supercapacitors based on metal hydroxide. Finally, we will discuss the potential future directions for EC research. We believe that the topic of this review will be interesting to scientists working in related fields.
2. Single Co or Ni hydroxide
2.1 Single crystalline hydroxide
Single crystalline metal hydroxide as an electrode material for ECs has been reported and investigated in many published papers, typically for transition metal hydroxides containing elements including nickel, cobalt, and iron. These metal hydroxides are often layered materials with a large interlayer spacing, and they can have very high theoretical specific capacitance. We focus firstly on interesting results reported using single metal (Co and Ni) hydroxide as electrode materials of ECs. | ||
| Fig. 1 Pictures depicting a flexible graphite screen printed electrode. Taken from ref. 20 with permission from RSC Publications. | ||
It is known that the supercapacitive performance of electrode materials strongly depends on their morphological creation, and thereby on the specific surface area. Thus, porous three dimensional (3D), hierarchical structures of Co(OH)2 such as coral-like shapes,23,24 flowerlike shapes,20,25−31 mesoporous nanowires,32−35 nanoflake films,36−42 nanocone arrays,43 and hollow core–shell structures were fabricated and developed for EC applications. The surfaces of these 3D Co(OH)2 structures show randomly distributed, interconnecting configurations, resulting in a network-like structure with many cavities between the adjacent blocks. The high specific surface area and numerous pores in the 3D Co(OH)2 can facilitate penetration by electrolytes, and thus contribute to the excellent capacitive properties. Thus, relevant methods have been developed for preparation of such electrode materials, including electrodeposition, hydrothermal method, liquid-precipitation, ionothermal synthesis,44 ball-milling,19 and sonochemical synthesis.15
Ionic liquids (ILs, 1-butyl-3-methylimidazolium tetrafluoroborate) and Co(OH)2 could form a nanohybrid with a large surface area of 400.4 m2 g−1 by ionothermal synthesis, as shown in Fig. 2. The IL–Co(OH)2 based electrode exhibited a higher specific capacitance of 859 F g−1 with a high-rate capability, better cycling performance, higher ion diffusion coefficient and lower charge transfer resistance than bare Co(OH)2.44 Theoretical calculations revealed that IL molecules consisting of anion and cation groups enabled an easier hydrogen desorption/adsorption process, leading to a favorable redox reaction on the Co(OH)2 surface.
 | ||
| Fig. 2 (a–d) TEM image of IL–Co(OH)2 (inset of image (d) shows a lattice image of individual IL–Co(OH)2); (e) XRD patters of IL–Co(OH)2, Co(OH)2, and [Bmim][BF4]; (f) nitrogen adsorption/desorption isotherms and pore size distribution of IL–Co(OH)2. Reprinted with permission from ref. 44. Copyright (2013) American Chemical Society. | ||
If Co(OH)2 films could be directly deposited on a current collector, the Co(OH)2 electrodes would have several advantages including not requiring the use of polymer binder and conducting agent. Therefore, preparation of Co(OH)2 films is another excellent route to obtain high-performance electrode materials.45 Among various synthetic methods, electrodeposition is popularly used. Li et al. compared various electrochemical tests of ordered mesoporous Co(OH)2 films on a foamed nickel and titanium plate.39 The Co(OH)2 film on Ni foam had a much higher specific capacitance (maximum: 2646 F g−1) than that on titanium plate (maximum 1018 F g−1) because of the larger surface area of Ni substrate. More recently, Salunkhe et al. applied a chemical deposition method to synthesize Co(OH)2 rod films on the current collector.46 The direct growth of Co(OH)2 rods gave an open-three dimensional structure for easy access of electrolyte throughout the material surface, being an efficient electrode for EC application. The electrode achieved the highest capacitance of 1116 F g−1 at a current density of 2 A g−1.
It is known that β-form Co(OH)2 has a brucite-like origin, where octahedral with divalent cobalt cations six-fold coordinated by hydroxyl ions share edges to produce 2D charge-neutral layers stacked one over the other without any anion. Most as-fabricated β-Co(OH)2 crystals have a lot of crystalline planes parallel stacking along the [001] direction. However, single-layer β-Co(OH)2 nanosheets can be prepared by phase transformation from exfoliating layered α-Co(OH)2 in formamide at 80 °C under nitrogen gas protection for 15 hours.47 The obtained β-Co(OH)2 nanosheets were measured with lateral size from several tens to several hundred nanometers, and thickness in the range of 0.9 to 1.3 nm. The selected area electron diffraction (SAED) image taken from an individual nanosheet corresponded to the crystalline structure of β-Co(OH)2, as shown in Fig. 3. The as-prepared single-layer β-Co(OH)2 nanosheets could be assembled with graphene oxide (GO) to form a two-dimensional composite. The reduced GO/β-Co(OH)2 composite exhibited a high specific capacitance up to 2080 F g−1 at a current density of 1 A g−1.
 | ||
| Fig. 3 (a) AFM and height profile, (b) TEM and SAED images of single-layer β-Co(OH)2 nanosheets. Reprinted with permission from ref. 47. Copyright (2014) American Chemical Society. | ||
Traditionally, the theoretical specific capacitance of inorganic pseudocapacitors can be calculated according to the transferred electric charge and weight of active electrode materials. The theoretical specific capacitance of active material can be calculated as Ct = (n × F)/(M × ΔV), where n is the moles of charge transferred per mole of active material, F is Faraday's constant, M is the molar mass of the electroactive phase, and ΔV is the operating voltage range.17a The theoretical value Ct of Co(OH)2 is 3460 F g−1 (within 0.6 V), assuming that all Co(OH)2 phase in the bulk is electrochemically accessible and contributes to the redox reactions.17a However, most reported experimental values are still lower than the theoretical values. The difficulty in approaching the theoretical specific capacitance is strongly associated with the limited ion diffusion within conventional dense electrode film, and poor electron transfer because of the semiconducting or insulating property of cobalt compounds, which means they do not take part fully in the electrochemical reactions. Thus, much effort still needs to be made to improve the electrochemical performance of Co(OH)2.
To obtain a high specific capacitance and good rate capability, nanoscaled Ni(OH)2 crystals were prepared owing to its high surface area and abundant pores. Ni(OH)2 nanosheets with high surface area and narrow pore size distribution could be synthesized facilely by a microwave assisted heating method, and were applied as electrochemical pseudo-capacitive materials for ECs,48 exhibiting a high specific capacitance of 2570 F g−1 at a current density of 5 A g−1 with good cycling stability. Shao et al. reported that rare metal La-doped nano nickel hydroxide was prepared by electrodeposition and it could reach the highest discharge capability of 840 F g−1.49
It is well accepted that porous structures can accelerate the diffusion of active species and facilitate electron transportation. 3D Loose-packed porous Ni(OH)2 for electrode materials is commonly prepared by various methods, including different morphologies such as coral-like50 microsphere of nanoflakes,51 snow-ball like,52-53 coin-like plates,54 flowery architecture,55,56 pompon-like,57 nanotube arrays,58 hollow microspheres,59 and nanowire aggregations.60 These porous structured Ni(OH)2 with low densities and high surface areas have thus attracted much attention not only for their importance in achieving a better understanding of the formation process but also because of their numerous potential technical applications, such as ECs.
To date, many attempts have been made on the synthesis of 3D nano- and micro-structured hollow Ni(OH)2. Some metal oxide nanoarrays (such as ZnO) can be used as a template to fabricate hollow Ni(OH)2 nanotube electrode material, as shown in Fig. 4. The opening of the Ni(OH)2 nanotube arrays provides many channels that facilitate penetration of the electrolyte and ions deep into the electrode, leading to an increase in surface area for electrochemical reaction.58,61
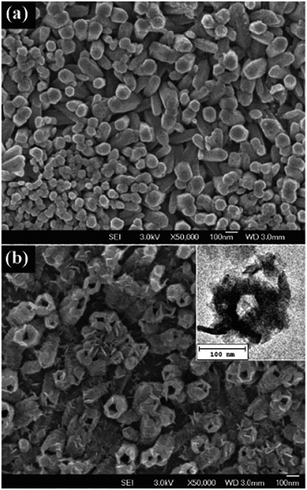 | ||
| Fig. 4 SEM micrographs of (a) ZnO nanorod arrays and (b) nickel hydroxide nanotube arrays after etching with 6 M NaOH. Inset in (b) is a top-view TEM micrograph of the nickel hydroxide nanotube. Taken from ref. 58 with permission from RSC Publications. | ||
Because 3D mesoporous nanostructure films can enhance supercapacitive performance by reducing the diffusion resistance of electrolytes and enhancing ion and electron transportation,62 ultrathin Ni(OH)2 nanowall films deposited on Ni foam with an ultrahigh capacitance of 2675 F g−1 and up to 96% reversibility were reported by Sun et al.,63 as shown in Fig. 5. This specific capacitance is beyond the theoretical value of β-Ni(OH)2 (2358 F g−1 within 0.44 V), and perhaps can be attributed to the combination of the Faradic capacitance from chemical reactions and the EDLC from the high surface area. A much higher specific capacitance observed on a Ni(OH)2–nickel foam composite was 3152 F g−1 at a current density of 4 A g−1,64 where loosely packed nanometer scale Ni(OH)2 grains spread on nickel foam maintained a greater surface area for reaction and resulted in effective utilization of electrode material. Both α-Ni(OH)2 and β-Ni(OH)2 films can be electrodeposited directly on nickel foam at different temperatures. The sample synthesized at 65 °C possessed a porous honeycomb-like structure and the highest specific capacitance was up to 3357 F g−1 at a current density of 4 A g−1.65 The above reports reveal the possibility of exceeding the theoretical Faradic capacitance of Ni(OH)2. These Ni(OH)2 crystal films usually have a 3D porous structure, ultrathin morphology, and direct connection to conductive substrates. In addition to electrodeposition,66−69 other methods such as chemical bath deposition,70−72 and hydrothermal methods73−78 were reported to controllably fabricate thin Ni(OH)2 films and they were applied as the electrode materials of ECs with a high specific capacitance.
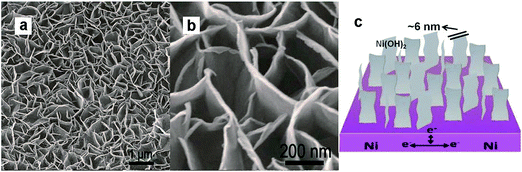 | ||
| Fig. 5 (a and b) Low- and high-magnification SEM of Ni(OH)2 nanowall films; (c) schematic image of Ni(OH)2 nanowall films. Adapted from ref. 63 after modification with permission from RSC Publications. | ||
Lokhande et al. found that Ni(OH)2 thin films with different nanostructures such as nanoplates, stacked nanoplates, nanobelts, and nanoribbons could be controllably fabricated by varying the deposition temperature.78 These electroactive Ni(OH)2 films grown on conductive substrates can be directly used as binder-free electrodes, and such electrode design has two advantages over conventional thin film electrodes: (1) the poorly intrinsic conductivity of the electroactive material is no longer a concern because of the direct attachment of electroactive materials to the conductive substrate; (2) this design makes auxiliary components like conductive agents and binders completely unnecessary. Thus, Ni(OH)2 films in situ deposited on a conductive substrate have great potential for EC application.
Although Ni(OH)2 has a lower theoretical specific capacitance than Co(OH)2, the reported specific capacitance values of Ni(OH)2 are usually much higher when compared with Co(OH)2. We assume that the formed structure and morphology of Ni(OH)2 are more suitable for electrochemical reaction than those of Co(OH)2. Considering that Ni is much cheaper than Co, Ni(OH)2 is thus a promising electrode material for ECs.
2.2 Amorphous monometallic hydroxide
Use of a metal hydroxide with poor crystallinity or amorphous phase may result in more transportation channels than use of a highly crystalline one, therefore some reports have been published on the fabrication and electrochemical properties of amorphous metal hydroxides. Tong and co-workers carried out some research on amorphous nickel hydroxides and cobalt hydroxides.79a They used electrochemical methods to prepare amorphous nickel hydroxide nanospheres (as shown in Fig. 6) exhibiting a high specific capacitance (2188 F g−1). Asymmetric pseudocapacitors made of the amorphous nickel hydroxide had a high capacitance (153 F g−1), a high energy density (35.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W h kg−1 at a power density of 490
W h kg−1 at a power density of 490![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W kg−1), and super-long cycle life (97% and 81% charge retentions after 5000 and 10
W kg−1), and super-long cycle life (97% and 81% charge retentions after 5000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, respectively).79a Then the Cao group synthesized amorphous mesoporous Ni(OH)2 nanoboxes with uniform size of 450–500 nm by a template-engaged route.79b The nanoboxes showed high specific capacitance of 2495, 2378, 2197, 1993 F g−1 at discharge currents of 1, 2, 5 and 10 A g−1, respectively.
000 cycles, respectively).79a Then the Cao group synthesized amorphous mesoporous Ni(OH)2 nanoboxes with uniform size of 450–500 nm by a template-engaged route.79b The nanoboxes showed high specific capacitance of 2495, 2378, 2197, 1993 F g−1 at discharge currents of 1, 2, 5 and 10 A g−1, respectively.
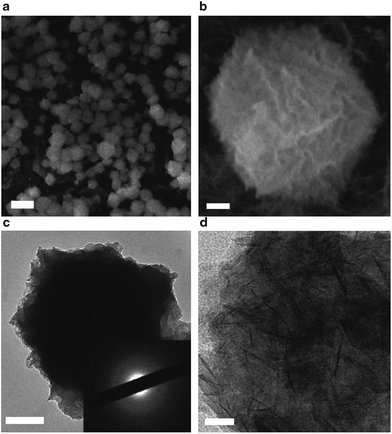 | ||
| Fig. 6 (a and b) SEM images of amorphous Ni(OH)2 samples synthesized on graphite electrodes. (c and d) TEM images of amorphous Ni(OH)2 samples (the inset shows the corresponding selected-area electron-diffraction pattern). Scale bars, 1 μm (a), 100 nm (b), 0.2 μm (c) and 20 nm (d). Reprinted with permission from ref. 79a. Copyright (2013) Nature Publishing Group. | ||
Amorphous Co(OH)2 nanostructures with excellent electrochemical behaviors can be synthesized on graphite flakes by a simple and green electrochemical method in deionized water without using any chemical additives. These amorphous cobalt hydroxides having three-dimensional structure possessed a high specific capacitance to 1094 F g−1 at a scan rate of 1 mV s−1, with specific capacitance loss of only 5% after 8000 consecutive cycles at 100 mV s−1.80 Integrated electrochemical performances of the amorphous hydroxide were totally commensurate with those of Co(OH)2 materials with crystalline phase.
2.3 Composites containing Ni hydroxides, Co hydroxides, and carbon materials
The major issues for metal hydroxides (Ni hydroxides and Co hydroxides) are their poor electrical conductivity and volume expansion during charge–discharge cycles, especially for high-rate applications. Therefore, considerable work has been performed to improve their electrochemical properties by tuning their morphologies at the nanoscale and modifying them to be nanocomposites with some conductive materials. An efficient method is to make a hybrid containing both a metal hydroxide and a conductive material such as graphene, carbon black, and carbon nanotubes. For these carbon materials, their capacitance primarily relies on an electrical double-layer mechanism without involvement of the Faradic process. Therefore, despite their high charge–discharge cycling stability and power density, most carbon materials still suffer from lower specific capacitance than transition metal hydroxides or oxides. To further increase the energy density of hydroxide materials, several kinds of carbon materials have been selected and incorporated into metal hydroxides to form pseudocapacitive composites.Although Co(OH)2 is considered to be an important transition metal hydroxide for ECs, it displays less satisfactory electrochemical capacity and reversibility compared with ruthenium oxides. Many composites containing Co(OH)2, such as Co(OH)2/carbon,81 and Co(OH)2/graphene82,83 have been prepared, with according improvement in electrochemical properties. The utilization percentage of Co(OH)2 increases in the composite, the carboneous support of Co(OH)2 yields a high conductivity and supports improvement of rate capability. So, a composite consisting of Co(OH)2 and carbon support will have greatly improved capacitance value.
Graphene (or reduced GO) is a novel carbon material with 2D structure, and has attracted intense interest in ECs electrode because of its unique electrical and mechanical properties. Addition of reduced GO (rGO) sheets is expected to provide a large support surface area for metal hydroxide nanocrystals and to improve conductivity of the resultant composite materials. Zhu et al. prepared a graphene/Co(OH)2 composite employing Na2S solution as both the depositing and the reducing agent.84 The composite had a remarkable specific capacitance of 972.5 F g−1, leading to a significant improvement in relation to each individual counterpart (137.6 and 726.1 F g−1 for graphene and Co(OH)2, respectively). It is possible that aggregation occurs, resulting in lower utilization of Co(OH)2 active material in the case of being dried. Decoration of graphene nanosheets (GNS) with Co(OH)2 nanoparticles could effectively inhibit the aggregation, resulting in higher utilization of Co(OH)2 and improved electrochemical performance. The specific capacitance of the rGO/Co(OH)2 composite prepared by solution method (as shown in Fig. 7) can reach 474 F g−1 at a current density of 1 A g−1 and this value can even retain 300 F g−1 at a high current density of 10 A g−1, showing a relatively good rate capability. Meanwhile, the specific capacitance of the electrode remained at 90% after 1000 times of cycling, showing good cycle stability.85 All above reports indicate that the enhancement of capacitive performance can be attributed to the synergistic effect between graphene and Co(OH)2 components in the composite.
 | ||
| Fig. 7 STEM image (a), and carbon (b), oxygen (c), and cobalt (d and e) element mapping images of rGO/Co(OH)2 composite. Reprinted with permission from ref. 85. Copyright (2013) Elsevier B. V. | ||
Ni(OH)2 is of particular interest as an EC materials because it is cheap and has high theoretical specific capacitance; however, one major drawback for Ni(OH)2 is its poor conductivity (∼10−17 S cm−1). A strategy to improve the electrochemical performance of Ni(OH)2 is to form a composite with an electronically conductive material. Many graphene/Ni hydroxide based composites have been successfully prepared, in which graphene or reduced GO improves the conductivity of the electrode materials.86−94 Single-crystalline Ni(OH)2 hexagonal nanoplates grown on graphene sheets showed a high specific capacitance (∼1335 F g−1 at a charge and discharge current density of 2.8 A g−1) and remarkable rate capability, significantly outperforming Ni(OH)2 nanoparticles grown on GO and Ni(OH)2 nanoplates simply mixed with graphene sheets,95 as reported by Dai and shown in Fig. 8. Many functional groups of GO (hydroxyl, epoxy, carboxyl, and carbonyl groups) can act as anchor sites for Ni(OH)2 crystal growth. Thus, a Ni(OH)2/graphene composite prepared by an electrostatic method showed superior electrochemical properties, including high specific capacitance (1503 F g−1 at 2 mV s−1) and excellent cycling stability up to 6000 cycles even at a high scan rate of 50 mV s−1.96 This was attributed to its tailored properties, which were vital to the operation of ECs, including intimate bindings, high conductivity, structural stability, and good wettability.
 | ||
| Fig. 8 SEM and TEM characterizations of Ni(OH)2/GS composite, Ni(OH)2/GO composite, and Ni(OH)2 + GS physical mixture. (a) SEM image of Ni(OH)2 nanoplates grown on GS. (b) TEM image of Ni(OH)2 nanoplates grown on GS. (c) SEM image of Ni(OH)2 nanoparticles grown on GO. (d) TEM image of Ni(OH)2 nanoparticles grown on GO. (e) SEM image of Ni(OH)2 hexagonal nanoplates grown in free solution (without graphene). (f) SEM images of simple physical mixture of presynthesized free Ni(OH)2 nanoplates and GS. Reprinted with permission from ref. 95. Copyright (2010) American Chemical Society. | ||
In addition to graphene and reduced GO, carbon nanotubes (CNTs) are also suitable conductive supports for Ni(OH)2 material.97 Holze and co-workers developed a “bottom-up” chemical method to coat nanocrystalline Ni(OH)2 onto the outer surface of multiwalled CNTs (MWCNTs) for flexible EC electrodes, where the high electronic conductivity of CNTs permitted their use as the supporting backbone onto which Ni(OH)2 could be deposited,98 as exhibited in Fig. 9. The high capacitance and excellent rate capability of Ni(OH)2/CNT electrodes could be attributed to the unique structure of the materials, where sponge-like Ni(OH)2 nanoparticles were supported on a high-packing density, porous multiwalled CNT network with adequate access to electrons and ions in the electrolyte. Ni(OH)2/CNT composites also can be prepared using an electrochemical deposition method. The specific capacitance of the nanocomposite Ni(OH)2/CNT electrode was as high as 2486 F g−1 and stable over long cycling.99 Meanwhile, active carbon also can be used to combine with Ni(OH)2 to improve electrochemical performance.100
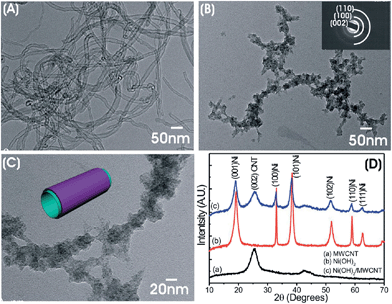 | ||
| Fig. 9 TEM images of (A) pristine MWCNT and (B and C) Ni(OH)2/MWCNT (30 mg cm−2) and (D) XRD patterns of MWCNTs, Ni(OH)2, and Ni(OH)2/MWCNT powders. Reprinted with permission from ref. 98. Copyright (2013) American Chemical Society. | ||
Zhao et al. first demonstrated preparation of 3D CNT-pillared rGO sheets with embedded Ni(OH)2 nanoparticles for pseudocapacitor applications. The composite displayed specific capacitances as high as 1235 and 780 F g−1 at current densities of 1 and 20 A g−1, respectively.101 Graphene/Ni(OH)2 composites also can be prepared by a scalable solid-state reaction method. The resulting composites with well-dispersed Ni(OH)2 nanoparticles on rGO surface showed a high specific capacitance of 1568 F g−1 and good cycling stability (>75% retention for 1000 cycles) at a current density of 4 A g−1 in 1 M KOH.102
3. Bimetallic hydroxide
Nickel hydroxide is deemed one of the most promising electrode materials owing to its high theoretical specific capacitance, up to 2358 F g−1. However, such high capacitance is hard to realize in practice owing to its poor conductivity. It has been reported that incorporation of cobalt into nickel hydroxide can significantly improve the electrochemical and conducting properties of the overall electrode material, because of formation of highly conductive CoOOH during the charge–discharge process.103 Thus, various nickel–cobalt double hydroxide electrodes have been prepared, and their enhanced electrochemical performances also have been reported.104−1213D porous Ni–Co binary hydroxides have been studied intensively because of their high specific surface area and porous structure. Urchin-like Ni(OH)2–Co(OH)2 hollow microspheres can be synthesized by a microwave-incorporated hydrothermal method.122 The hollow microspheres achieved a high specific capacitance of 2164 F g−1 at 1 A g−1 and long-term cycle life. Co0.5–Ni0.5 hydroxide nanocones can deliver a specific capacitance of 1580 F g−1 at a galvanic current density of 10 A g−1, measured from charge–discharge curves. The authors attributed this to enhancement of electroactive sites participating in the redox reaction because of possible valence interchange or charge hopping between Co and Ni cations.106 Hollow rhombic dodecahedral NiCo hydroxide nanocages, composed of thin nanoplatelets, can be synthesized using ZIF-67 nanocrystals as templates. The porous nanocages exhibited superior pseudocapacitance properties because of their novel hierarchical and submicroscopic structures.111,113
Ni–Co double hydroxide films also show great potential for EC electrodes. A high specific capacitance of 2682 F g−1 at 3 A g−1 based on active materials was obtained using Ni–Co layered double hydroxide (LDHs) hybrid film on nickel foam.104 Co0.72Ni0.28 double hydroxides deposited on stainless steel by a potentiostatic deposition method had the highest specific capacitance of 2104 F g−1 in 1 M KOH.114 The composition ratio of Ni–Co had great influence on the morphology of double hydroxide arrays deposited on stainless steel, where Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co = 2
Co = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 achieved a specific capacitance of 456 F g−1 with an energy density of 12.8 W h kg−1.109 The nanocrystalline Co1−xNix hydroxide thin films prepared by potentiodynamic deposition possessed different porous, nanoflake like morphology and superhydrophilic behavior by the composition influence. The maximal specific capacitance for Ni–Co hydroxide electrodes was found to be about 1213 F g−1 for the composition Co0.66Ni0.34 LDHs in 2 M KOH electrolyte at 5 mV s−1 scan rate.116
1 achieved a specific capacitance of 456 F g−1 with an energy density of 12.8 W h kg−1.109 The nanocrystalline Co1−xNix hydroxide thin films prepared by potentiodynamic deposition possessed different porous, nanoflake like morphology and superhydrophilic behavior by the composition influence. The maximal specific capacitance for Ni–Co hydroxide electrodes was found to be about 1213 F g−1 for the composition Co0.66Ni0.34 LDHs in 2 M KOH electrolyte at 5 mV s−1 scan rate.116
Zwitterionic p-aminobenzoate intercalated α-hydroxides of nickel and cobalt can be synthesized by ammonia precipitation, and have been shown to exfoliate in water. The monolayers from a mixture of aqueous colloidal dispersions of the α-hydroxides can be co-stacked instantaneously by addition of nitrate anions to give hybrids in which the two hydroxide layers were interstratified, as shown schematically in Fig. 10. The hybrid hydroxides having 80% nickel hydroxide showed the best electrochemical performance with a high specific capacitance of 990 F g−1 and very good cycling stability.123
 | ||
| Fig. 10 Schematic representation of the preparation of the nitrate intercalated interstratified α-hydroxide hybrids. Reprinted with permission from ref. 123. Copyright (2013) Elsevier Ltd. | ||
Ni–Co double hydroxides deposited on ZnO nanowire arrays108 and zinc tin oxide nanowires107 demonstrated outstanding performances with specific capacitances of 1624 and 1805 F g−1, respectively. Li et al. also found that the atomic ratio between Co and Ni had a significant effect on their electrochemical activities. A specific capacitance of 2614 F g−1 was achieved when the atomic ratio of Co to Ni was 0.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.43.110 Ni–Co binary hydroxide systems with controllable morphologies from nanosheets, to nanoplates-nanospheres, to nanorods, and to a nanoparticle geometry by simply tailoring the Ni and Co cation ratios in the initial reactants were reported by Lian.124 The drastically different morphologies significantly affected the electrochemical performance of the binary hydroxides as electrode materials. A high capacitance of 1030 F g−1 was achieved for the nanorod morphology at a current density of 3 A g−1.124 Even the lattice spacing and crystal size of Co–Ni hydroxide nanosheets could be slightly tuned by the metal ratio.125 Although the optimum Co/Ni ratios are different in the above reports because of different preparation methods, we can still draw a conclusion that, to a large extent, bimetallic hydroxides of Ni–Co are superior to their individual components.
0.43.110 Ni–Co binary hydroxide systems with controllable morphologies from nanosheets, to nanoplates-nanospheres, to nanorods, and to a nanoparticle geometry by simply tailoring the Ni and Co cation ratios in the initial reactants were reported by Lian.124 The drastically different morphologies significantly affected the electrochemical performance of the binary hydroxides as electrode materials. A high capacitance of 1030 F g−1 was achieved for the nanorod morphology at a current density of 3 A g−1.124 Even the lattice spacing and crystal size of Co–Ni hydroxide nanosheets could be slightly tuned by the metal ratio.125 Although the optimum Co/Ni ratios are different in the above reports because of different preparation methods, we can still draw a conclusion that, to a large extent, bimetallic hydroxides of Ni–Co are superior to their individual components.
Liu et al. developed an accumulative approach to move beyond simple incorporation of conductive carbon nanostructures to improve performance of metal hydroxides. Ni–Co double hydroxide/graphene composites were first synthesized by co-precipitation, and then assembled into films by integrating with few walled CNTs that could be directly used as electrodes, as shown in Fig. 11. With 50% Co and 50% Ni, the composite exhibited a remarkable maximum specific capacitance of 2360 F g−1 at 0.5 A g−1. The control experiments showed that the double hydroxides outperformed either Co(OH)2 or Ni(OH)2 alone,126 proving that graphene can greatly boost electron transfer during the redox reaction.127
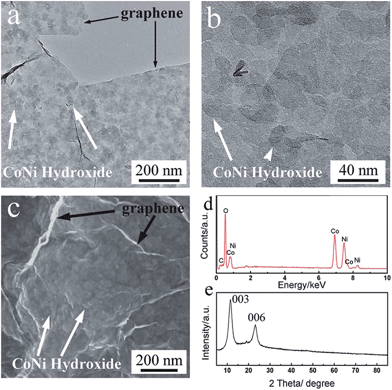 | ||
| Fig. 11 Material characterization of the Co0.5Ni0.5(OH)2/graphene composite: (a) and (b) TEM images at different magnifications; (c) SEM image; (d) EDS spectrum and (e) XRD pattern. Taken from ref. 126 with permission from RSC Publications. | ||
More recently, Ni–Cu double hydroxide spheres were synthesized by a chemical bath deposition method on different substrates including copper foam, nickel foam, and carbon paper. When the substrate was changed from a copper foam to a nickel foam or carbon paper, the deposited material became pure nickel hydroxide with a flower-like morphology. The Ni–Cu double hydroxide also demonstrated a high specific capacitance of 1970 F g−1.128
4. Hydrotalcite-like compounds
4.1 Layered double hydroxides
Layered double hydroxides (LDHs) are a kind of lamellar compound made of positively charged brucite-like layers and an interlayer region containing compensating charge anions and salvation molecules. The most widely studied LDHs usually contain both divalent and trivalent metal cations. A general formula for LDHs can be written as, [M2+1−xM3+x(OH)2][An−]x/n·zH2O, where M2+ may be common, such as Mg2+, Zn2+, Co3+, or Ni2+, and M3+ may be Al3+, Ga3+, Fe3+, or Mn3+. An− is a charge compensating inorganic or organic anion, e.g. CO32−, Cl−, SO42−, and x is normally between 0.2 and 0.4. As a result of the flexible ion-exchangeability and easily tunable composition, LDHs have a series of important applications in catalyst or catalyst precursors, anion exchangers, precursors to other materials and so on. Some earth-abundant transition elements including Co, Ni, Mn and Fe, can be used as the building blocks of LDH layers and there are wide and flexible galleries between host layers. These LDHs also have been applied as electrode materials in ECs as a result of the LDHs nanosheets facilitating increased surface area and remarkably decreased diffusion distances for ions.129 The simplest and most common method for preparation of LDHs is co-precipitation of the chosen M2+ and M3+ hydroxides with diluted NaOH and/or Na2CO3 or NaHCO3. However, they can also be synthesized using urea hydrolysis, hydrothermal or ion-exchange methods. Nickel and cobalt are the two most commonly studied elements in these systems because of their abundance and high capacitance.Excellent capacitive materials should have large surface area and highly ordered dimensions. There are three important factors in realization of these goals. Firstly, larger specific surface area of active materials can accommodate more electrolyte ions for redox processes. Secondly, providing a suitable mesopore structure is important for mass transfer of electrolytes. Finally, electroactive materials with high electrical conductivity can deliver high capacitance under a high discharge current density, exhibiting an excellent high rate capability. Based on this, these factors have been intensively investigated by scientists. Mousty el al. found that the electrochemical behavior of Ni-rich LDH was mainly governed by an electron hopping mechanism, whereas in CoAl-LDH a multi-site pseudocapacitive behavior was observed.130
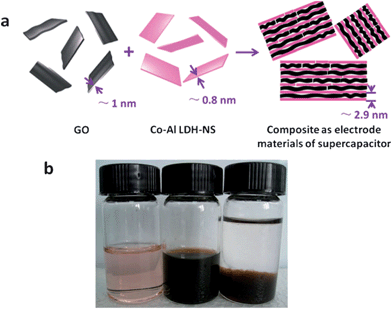 | ||
| Fig. 12 (a) Schematic of the formation and structure of Co–Al LDH-NS/GO composite. (b) Digital photographs of (left) an aqueous dispersion of Co–Al LDH-NS, (middle) an aqueous dispersion of GO, and (right) a mixture of Co–Al LDH-NS and GO. Taken from ref. 134 with permission from RSC Publications. | ||
However, when CoAl LDHs were soaked in KOH solution, Al3+ ions were leached off from the lattice, transforming them into β-Co(OH)2, and degrading the electrochemical performance of the electrode. The transformations were influenced by the CoAl ratio, soak temperature, alkali concentration, and also took place during the charge–discharge process.136 The dissolution of Al(OH)3 in the electrolyte and/or surface modification of Y, Er, or Lu can improve the cycling charge–discharge performance because they can retard transformation from LDH into β-Co(OH)2.
CoAl LDHs usually suffer from low rate and poor cycling stability at high current densities. One strategy to deal with these drawbacks is doping LDHs with other carbon materials. Coating CoAl LDHs with conductive film or self-assembling them onto graphene or other conductive supports can greatly improve the high-current capacitive behavior.137−139 Formation of CoAl LDHs on the graphene nanosheets can prevent restacking of the as-reduced graphene nanosheets,140,141 as depicted in Fig. 13. Zhang et al. applied a refluxing method with urea as a basic precipitant for 48 hours to prepare graphene/CoAl-LDH, where the CoAl-LDH crystals adhered to the surface of graphene nanosheets to form a laminated structure with smaller size compared with the pure CoAl-LDH, which facilitated electrolyte soaking into electrode materials.139 A time saving method is microwave-assisted irradiation; the reaction time can be reduced greatly to about 2 hours.142
 | ||
| Fig. 13 (a) Low and (b) high magnification TEM images of nanosized LDHs/GO hybrid at high LDH nuclei concentration. Reprinted with permission from ref. 140. Copyright (2012) American Chemical Society. | ||
A CoAl-LDH@poly(3,4-ethylenedioxythiophene)(PEDOT) core–shell nanoarray was grown on a flexible Ni foil substrate.138 Its performances were superior to those of conventional LDH arrays without the PEDOT coating. The enhanced pseudocapacitor behavior of the LDH@PEDOT electrode was related to the synergistic effects of its individual components. The LDH nanoplatelet core provided abundant energy storage capacity, while the highly conductive PEDOT shell and porous architecture facilitated electron/mass transport in the redox reaction.138 Pt film-coated CoAl LDH arrays also exhibited enhanced electrochemical properties.137
Core–shell NiAl LDH microspheres with tunable interior architecture have been fabricated and reported by a facile and cost-effective in situ growth method on Si microspheres, as presented in Fig. 14. The hollow NiAl-LDH microspheres with the highest surface area (124.7 m2 g−1) and a mesopore distribution (3–5 nm) can give a maximum specific capacitance of 735 F g−1 and good cycle performance, as well as remarkable rate capability.148
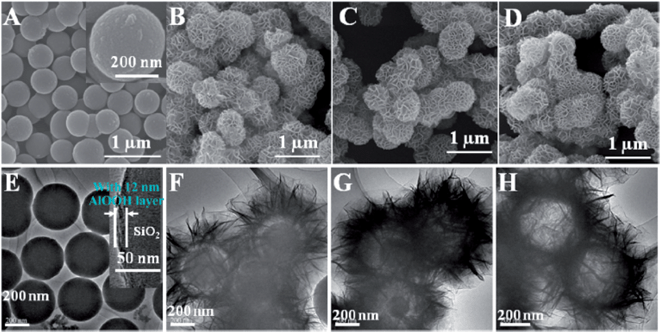 | ||
| Fig. 14 TEM and SEM images of (A and E) SiO2/AlOOH microspheres; (B and F) SiO2/NiAl-LDH core–shell microspheres; (C and G) SiO2/NiAl-LDH yolk–shell microspheres; (D and H) NiAl-LDH hollow microsphere. Reprinted with permission from ref. 148. Copyright (2012) American Chemical Society. | ||
Although capacitance of up to 1000 F g−1 has been reported for NiAl LDHs in various electrolytes, they often suffer from low electrical conductance and short cycle life during application. Thus, some efforts have been made to improve their performance by creating conductive nanostructure-supported NiAl LDHs,149−155 as shown in Fig. 15. Incorporation of NiAl LDH platelets onto graphene can prevent restacking of graphene nanosheets (GNS) and improve capacitance of the composite electrode, the electrolyte/electrode accessibility, and the conductivity.156,157 The prepared GNS/LDH composite exhibited a high specific capacitance (781.5 F g−1 at 5 mV s−1) and excellent long cycle life.158 Using GNS as a support, a GNS/NiAl-LDH composite was prepared successfully by a liquid phase deposition method as an electrode material for ECs. Because of the improvement of conductivity and adhesion, facile electrolyte penetration, and better Faradic utilization of the electroactive porous surface, the as-obtained GNS/NiAl-LDH composite showed a considerably improved electrochemical performance with a specific capacitance of 1255.8 F g−1.159 An alternative method was use of layer-by-layer deposition of AlOOH onto GNS followed by an in situ growth process of NiAl-LDHs to form an interesting sandwich structure,160,161 with the composite having a specific capacitance of 1329 F g−1 at a current density of 3.57 A g−1.
 | ||
| Fig. 15 (A) SEM image of Ni foam; (B) SEM image and digital photo (inset in (B)) of NiOOH; (C) small and large (inset in (C)) SEM images of DOS-LDH; (D and E) SEM images and digital photo (inset in (D)) of NiAl-LDH; (F) lateral view image of NiAl-LDH. Reprinted with permission from ref. 151. Copyright (2013) Elsevier B.V. | ||
As reported by Wimalasiri,162 delaminated NiAl-LDHs also can be incorporated between graphene nanosheets to form a layered hybrid structure as a new supercapacitor electrode material, GO and delaminated NiAl-LDH in aqueous medium, rather than in situ growth of NiAl-LDH on GO nanosheets. Both materials in their layered form provide more contact between electrochemically active areas and result in favorable conditions to realize their electrochemical energy storage capacities. The graphene/NiAl-LDH-based electrodes provided a specific capacitance of 915 F g−1 at a current density of 2 A g−1.162
Similar to CoAl-LDHs, when a layered double hydroxide [Ni4Al(OH)10]NO3 was aged at 60 °C in a concentrated KOH solution (around 7 mol L−1), it transformed into β-form Ni(OH)2; when it was cycled electrochemically, it could also transform into β-Ni(OH)2 but finished much sooner, resulting in decrease of the maximum number of exchanged electrons per nickel atom as the charging–discharging cycling continued and degradation of the electrodes. The well-crystallized β-Ni(OH)2 had a worse electrochemical activity than NiAl-LDHs; however, its high temperature performance could be improved by lowering the concentration of electrolyte and/or adding rare metal oxides such as Lu2O3, Er2O3, or Y2O3.163
CoMn-LDH nanowalls were deposited onto flexible carbon fibers (CF) via an in situ growth approach. The resulting CoMn-LDH/CF electrode could deliver a high specific capacitance (1079 F g−1 at 2.1 A g−1 normalized to the weight of the active LDH material) with excellent rate capability even at high current densities (82.5% capacitance retention at 42.0 A g−1).166 The dramatic performance is mainly attributed to the homogeneous and ordered dispersion of metal units within the LDH framework, which enriches the redox reactions associated with charge storage by both Co and Mn.
Magnetic films of CoFe-LDH nanoplates and porphyrin anions were fabricated by the layer-by-layer technique, with the assistance of an external magnetic field, and showed enhanced electrochemical behavior.167 The Hwang group reported ZnCo-containing LDH phases and their exfoliated and calcined derivatives. The ZnCo-LDH materials showed not only interesting magnetic properties but also pseudocapacitance behavior with a discharge capacity of 160–170 F g−1 and a good capacitance retention.168
A NiTi-LDH thin film on nickel foam substrate was synthesized using two hydrothermal treatment steps with ammonia solution as a basic precipitant. NiTi-LDH crystallites perpendicular to the surface of the nickel foam substrates were formed and Ti atoms were dispersed homogeneously in the LDH lattice. A high specific capacitance of 10.37 F cm−2 was achieved at a current density of 5 mA cm−2 and 86% of the initial specific capacitance remained after 1000 cycles.169 NiV-LDH composites were prepared by a co-precipitation process, during which small amounts of vanadium sources were decomposed by acid solution and mixed with β-Ni(OH)2, and then a small quantity of nickel hydroxides transformed gradually to NiV-LDH composite. Compared with flake-like β-Ni(OH)2, the NiV–LDH composite exhibits a much higher specific capacitance of 2612 F g−1 at a scan rate of 2 mV s−1.170
Other LDHs such as CoCr- and CoIn-LDHs also have been investigated as electrode materials of ECs with good stability; however, their specific capacitances were not high.171
A hierarchical structure composed of NiMn-LDH microcrystals grafted onto a CNT backbone was prepared by Zhao and colleagues by chemical deposition,172 as shown in Fig. 16. The unique NiMn-LDH/CNT core–shell heterostructure can be seen in SEM and TEM images. The NiMn-LDH played the role of electrochemically active species, while CNTs served as both support and electron collector. Electrochemical measurement showed that the Ni3Mn1-LDH/CNT electrode was rather active, delivering a maximum specific capacitance of 2960 F g−1 (at 1.5 A g−1), together with good rate capability and excellent cyclability. Flexible ECs with asymmetric configuration can be fabricated using NiMn-LDH film and rGO/CNTs film as the positive and negative electrode, respectively, exhibiting a wide cell voltage of 1.7 V and large energy density of 88.3 W h kg−1.172
 | ||
| Fig. 16 (a) SEM image of the pristine CNT (inset: the enlarged image). (b and c) SEM images and (d and e) TEM images of the NiMn-LDH/CNT. (f) XRD patterns of: (i) the pristine CNT, (ii) the powdered sample of NiMn-LDH, (iii) the NiMn-LDH/CNT. Reprinted with permission from ref. 172. Copyright (2014) Wiley-VCH. | ||
CoNiAl three-component LDHs with hydrotalcite-like structure can be synthesized successfully by homogenous precipitation using urea as a precipitant, whose capacitive behavior was influenced by the Co/Ni atomic ratio and electrolyte. The CoNiAl-LDH displayed the best capacitive performances when the molar ratio of Co/Ni reached 5.173 The Co0.55Ni0.13Al0.32-LDH delivered more specific capacitance in 1 M KOH than in 1 M LiOH or NaOH. Increasing the concentration of KOH from 1 to 6 M, the specific capacitance was elevated from 644 to 1124 F g−1 at 1 A g−1. After 1000 continuous charge–discharge cycles at 2 A g−1, Co0.55Ni0.13Al0.32-LDH exhibited a good capacitance retention (93.3%).173 When ternary-component NiCoAl-LDH nanosheets and CNT composites were fabricated using urea precipitation, the small size CNTs were incorporated into the network of LDH nanosheets to form a homogeneous hybrid, as shown in Fig. 17. The specific capacitance can reach 1035 F g−1 at a current density of 1 A g−1, and increase by 33.3% in comparison with that of pure NiCoAl-LDH nanosheet.174 In addition to precipitation, NiCoAl-LDH nanosheets with anisotropic morphology also can be synthesized by potentiostatic deposition, and their composition can be changed easily.175
 | ||
| Fig. 17 FE-SEM and TEM images of (a and b) the NiCoAl-LDH nanosheets and (c and d) the NiCoAl-LDH–MWCNT nanohybrids, and high-resolution TEM images of (e and f) the NiCoAl-LDH–MWCNT nanohybrids. Taken from ref. 174 with permission from RSC Publications. | ||
Well-dispersed LiAl-LDHs can be synthesized by a solvothermal approach by adjusting the concentration of ethanol. The hexagonal LiAl-LDHs calcined at 450 °C exhibited specific capacitance of 848 F g−1 at a current density of 1.25 A g−1.176
4.2 α-Cobalt hydroxide
Cobalt hydroxide is well known to crystallize in two polymorphs, i.e. the metastable α-type and thermodynamically stable β-form. The first is isostructural with a hydrotalcite-like structure consisting of positively charged Co(OH)2−x(OH)x layers and charge balancing anions in the spaces between hydroxide layers. This structure is superior to that of the β-form for electrochemical reactions because it is a poorly and turbostratically crystallized structure, with large interlayer spacing. α-Type Co(OH)2 materials used for EC can be prepared by co-precipitation,177−181 hydrothermal method,182−185 electrodeposition,186−194 ionothermal synthesis,195 and γ-ray irradiation.196 A biomolecule-assisted method can be used to synthesize 2D nanoporous α-Co(OH)2 mesocrystal nanosheets, where the amino acid L-arginine plays a dual role. It acts as a hydrolysis-controlling agent while stabilizing the synthesized Co(OH)2 nanocrystallite building blocks.183The intercalated anions are exchangeable for α-Co(OH)2, thus making it possible to adjust the interlayer spacing. Through an anion exchange reaction, the interlayer spacing and intercalated anions in α-Co(OH)2 layers can be changed. Hu et al. reported that the intercalated anions in α-Co(OH)2 had a critical effect on basal plane spacing, morphology, and specific capacitance.197 Jin et al. also found that the interlayer space and electrochemical activity of α-Co(OH)2 were closely related and that larger interlayer spacing allowed more electrolyte ions to be stored, leading to higher electrochemical activity.198
However, the low conductivity of Co(OH)2 usually limits its high rate capabilities. The hybrid synthesis of α-Co(OH)2 on conductivity ITO nanowires forming nanoscale heterostructures can lead to improved high rate capabilities, originating from enhanced electrode and electrolyte conductivities, where ITO nanowires improve electron conductivity without using carbon black and polymer binder.199−201 Meanwhile, using α-Co(OH)2 and conductive carbon composites was also effective to improve electrochemical properties.191,193 CNTs grown on carbon substrates offer an external surface area for supporting electroactive α-Co(OH)2 materials, as presented in Fig. 18. Thus, nanometer-sized α-Co(OH)2 sheets were attached to CNTs/carbon paper substrates using an electrodeposition technique, as reported by Zhang et al.190 The composite electrode showed a large specific capacitance of 1083 F g−1 at a current density of 0.83 A g−1 in 6 M KOH, because the interconnected nanosheets of α-Co(OH)2 facilitated contact of active material with electrolyte. The CNTs provide a short path for ion/electron transfer and sufficient contact between materials and electrolyte.190
 | ||
| Fig. 18 SEM images of prepared Co(OH)2 deposited at different times of 180 s (a and b) and 300 s (c and d). Reprinted with permission from ref. 190. Copyright (2013) Elsevier Ltd. | ||
Another method that can be adopted is use of guest metal substitution to dope α-Co(OH)2, such as Al and Zn.179,189 Zn-substituted α-Co(OH)2 nanosheet electrodes exhibited much better cycling performance than the pure α-Co(OH)2 nanosheet electrode, as reported by He et al.189 The sample with 21.1 at.% Zn substitution demonstrated high cycling stability with a capacitance loss of only 0.6% from 652 F g−1 after 2000 cycles. Recently, exfoliation of α-Co(OH)2 in formamide solution was shown to form graphene-like 2D ultrathin α-Co(OH)2 nanosheets. The exfoliated α-Co(OH)2 ultrathin nanosheets had much better electrochemical properties than the precursor, including a high specific capacitance of 1280 F g−1, remarkable rate capability, and good cycling stability.180 The ultrathin nanosheets obtained by exfoliation are suitable for industrialization owing to the simple synthetic procedures and reaction conditions.202
However, α-Co(OH)2 is metastable in structure and easily undergoes a phase transformation into the more stable β-counterpart in strongly alkaline media. When immersed in KOH solution for a long time, this transformed to β-Co(OH)2 and Co3O4, with a dramatic decrease in specific capacitance.203 To keep α-Co(OH)2 stable in solution, inert atmosphere and weak alkaline media are necessary.
4.3 α-Nickel hydroxide
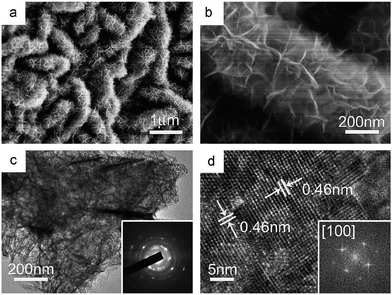
Similar to cobalt hydroxide, nickel hydroxide also has two polymorphs, i.e. α- and β-type Ni(OH)2. α-Hydroxides of nickel have a structure similar to LDHs. β-Type is of the formula Ni(OH)2 and is an ordered stacking of neutral layers of the compound with interlayer spacing of 0.46 nm. These have been studied extensively as they are widely employed as electrode materials of alkaline secondary batteries and supercapacitors.204Methods used for preparation of α-Ni(OH)2 as electrode materials of supercapacitors include electrochemical deposition,205−209 precipitation,210−212 and hydrothermal methods.213−217 Microwave heating is another very useful technique that has been widely applied for synthesis of inorganic compounds including α-Ni(OH)2. Mesoporous α-Ni(OH)2 material was reported to be produced on a large scale with assistance of 2.5 minutes of microwave irradiation in ethylene glycol solution.218
3D hierarchically structured α-Ni(OH)2 materials are good candidates for electrode of ECs; the porous features facilitate easy transportation of electrolyte to reaction sites and lead to a further increase in specific capacitance.219 Hollow spheres of α-Ni(OH)2 were fabricated by Wu via electrochemical deposition using a polystyrene sphere as template, and showed greatly enhanced electrochemical performance in alkaline solution because of its opening structure.207 Without requiring sacrificed templates, flower-like α-Ni(OH)2 microspheres composed of nanowires could be prepared by a solvothermal method using triethylene glycol and water as the mixed solvent. Compared with other conventional solvents, such as glycerol, alcohol, and water, triethylene glycol had a longer chain length, and moderate polarity and viscosity. Thus, the crystalline products obtained in this triethylene glycol/H2O solvent tended to have kinetically slower growth rate. The as-formed nanocrystals had enough time to rotate adequately and found the low-energy configuration interface, then growth units with oriented growth direction were formed. These building blocks coalesced and aggregated together to form porous microspheres, as shown in Fig. 19. The sample had a high BET surface area of 318 m2 g−1 and showed a high specific capacitance of 1788.9 F g−1 at 0.5 A g−1 as well as excellent rate performance.213
 | ||
| Fig. 19 (a and b) SEM and (c–e) TEM images of the microflower-like α-Ni(OH)2. Reprinted with permission from ref. 213. Copyright (2013) American Chemical Society. | ||
One simple method to improve the electrochemical properties of α-Ni(OH)2 materials is annealing them under a rational temperature. Porous flower-like α-Ni(OH)2 microspheres were synthesized by aqueous-phase reaction. Heating under a moderate temperature, i.e. 200 °C can deliver the highest specific capacity of 1551 F g−1 in 6 M KOH, while a higher calcination temperature would decrease the capacitance because of formation of NiO.220 Yang et al. reported that annealing α-Ni(OH)2 films on Ni foam at 100 °C delivered a specific capacitance of 2447 F g−1.221 It is supposed that the improved capacitance resulted from removal of physically absorbed water in α-Ni(OH)2. Yu et al. found that flower-like NiO/α-Ni(OH)2 composite had superior pseudocapacitive performance over individual α-Ni(OH)2 and NiO, but poor electronic conductivity hindered its capacitance retention at high current density, where the composite was obtained by heating α-Ni(OH)2 at 250 °C for different times. Another method that can be adopted is guest elemental doping in α-Ni(OH)2 crystals. Al and Zn doped α-Ni(OH)2 materials were reported to be prepared by electrochemical deposition and a hydrothermal method,222,223 tending to formation of Ni-based LDHs.
However, poor conductivity is the major drawback for Ni(OH)2 as a semiconductor material, leading to poor rate capability of the electrode, as well as low power output of the ECs. An efficient route to overcome this shortcoming is supporting Ni(OH)2 on carbon materials, of which CNTs are optimum.224−226 An α-Ni(OH)2/graphite nanosheet composite was prepared via a homogeneous precipitation method to form a 3D hierarchical porous structure, of which fine α-Ni(OH)2 nanocrystals as building blocks formed directly on the matrix of graphite nanosheets. The composite exhibited specific capacitance as high as 1956 F g−1 at a current density of 1 A g−1, and was able to endure the high discharge rate at current density of 40 A g−1.227 α-Ni(OH)2 nanosheets could be grown vertically on the surface of individual CNTs in CNT paper to form hierarchical nanowires by chemical bath deposition.228 This novel structure had a high electrochemical capacitance of 1144 F g−1 at a current density of 0.5 A g−1, and maintained 585 F g−1 at 10 A g−1. Flexible α-Ni(OH)2 nanofibers also can be intertwined and wrapped homogenously on polypyrrole-based carbon networks, leading to formation of complex networks. A specific capacitance of 1745 F g−1 could be obtained for Ni(OH)2/CNT composite at 30 mA cm−2.229 Uniform and conformal coating of α-Ni(OH) flakes on carbon microfibers was deposited in situ by a chemical bath method at room temperature. The microfiber-coated α-Ni(OH) flakes exhibited five times higher specific capacitance compared with non-conformal flakes, and the improvement was ascribed to the 3D network of the fibrous carbon fabric.230
Recently, graphene based composites were prepared by incorporating guest materials into 2D graphene sheets, for improving performance.231,232 rGO/CNTs/α-Ni(OH)2 composites were synthesized by a one-pot hydrothermal route. Electrochemical capacitance depended on the amount of CNTs, and the composite with optimized ratio exhibited high specific capacitance of 1320 F g−1 at 6 A g−1.233 Reduced GO/CNT formed a 3D conductive network in the composite, which promoted not only efficient charge transport and facilitated electrolyte diffusion, but also prevented effectively the volume expansion/contraction and aggregation of α-Ni(OH)2 during the charge–discharge process.233 Low defect density graphene-supported Ni(OH)2 sheets fabricated via a hydrothermal method were reported by Zhu et al.,234 where graphene simultaneously acts as both nucleation center and template for in situ growth of smooth and large-scale Ni(OH)2 nanosheets. The specific capacitance of the as-obtained composite is 1162.7 F g−1 at a scan rate of 5 mV s−1 and 1087.9 F g−1 at a current density of 1.5 A g−1. Meanwhile, there was no marked decrease in capacitance at a current density of 10 A g−1 after 2000 cycles.234 3D Porous graphene hollow sphere frameworks were fabricated by integrating GO with amino-modified SiO2 nanoparticles followed by etching, and were used as a support to combine with α-Ni(OH)2 nanoparticles, as exhibited in Fig. 20. These porous graphene hollow spheres had formed a continuous framework and there was a homogeneous coating of Ni(OH)2 throughout the 3D framework. The Ni(OH)2 exhibited a specific capacitance of 2815 F g−1 at 5 mV s−1. Increasing the scan rate to 200 mV s−1, α-Ni(OH)2 still maintained a specific capacitance of 1950 F g−1 with a capacitance retention of about 70%.235
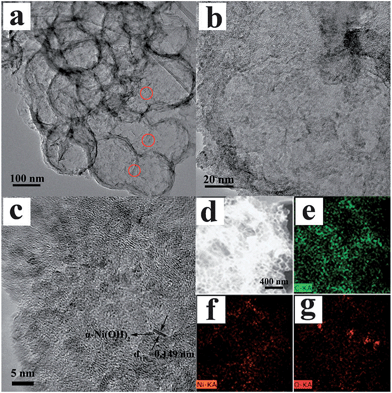 | ||
| Fig. 20 (a–c) TEM images at different magnifications and (d–g) STEM-mapping of porous graphene hollow spheres combined with α-Ni(OH)2. Reproduced from ref. 235 with permission from the PCCP Owner Societies. | ||
In addition to the carbon materials, ZnO is also a conductive candidate substrate for α-Ni(OH)2 crystals to anchor to make a binder-free electrode for ECs.236 Fan et al. employed ZnO nanowire electrode as a 3D framework to support large-area α-Ni(OH)2 growth and utilized ZnO nanowires with good electrical conductivity to provide a natural pathway for electron transport, as shown in Fig. 21.237 As each individual flake was connected to ZnO nanowire, the need for binders or conducting additives, which add extra contact resistance or weight, was eliminated. However, possible corrosion in a basic solution of ZnO might be one drawback after long cycling time.
 | ||
| Fig. 21 (a–c) General SEM images, (d) TEM image and (c) cross-sectional SEM images of the Ni hydroxide–ZnO hybrid structure, (f) Schematic diagram showing the kinetic advantage of the hybrid array in electrochemical energy storage. Taken from ref. 237 with permission from RSC Publications. | ||
5. Fe-based hydroxides
Compared with nickel and cobalt, iron is much cheaper and more abundant in the earth. Fe-based oxides and hydroxides (FeOOH) have also attracted great interest because of this natural abundance and their eco-friendliness, as electrodes of lithium ion battery and supercapacitors, in addition to acting as catalysts, adsorbents, and magnetic material.238Cheng et al. first confirmed that FeOOH was suitable for use as a negative electrode in hybrid ECs with an activated carbon positive electrode. The hybrid EC exhibited an estimated specific energy of 45 W h kg−1 based on the total weight of two electrodes with a good cycling performance, retaining 96% initial capacity after 800 cycles.239 Similarly, Jin also reported that a hybrid EC containing a nano-sized columned FeOOH negative electrode and MnO2 positive electrode, demonstrated an energy density of 12 W h kg−1 and a power density of 3700 W kg−1.240
Various morphologies of FeOOH materials have been fabricated and used as electrode materials of ECs.241−243 Marigold-like structured nickel doped (5 at.%) iron hydroxide thin film was deposited on stainless steel using electrodeposition, and used for EC application. The highest specific capacitance was 287 F g−1 in 1 M Na2SO3 electrolyte at a scan rate of 10 mV s−1.244 Large area self-standing γ-FeOOH nanosheets fabricated on iron foil were evaluated as electrodes of ECs in various electrolytes. These exhibited an areal capacitance of 0.3–0.4 F cm−2 and good cycling stability in Na2SO3 electrolyte, but were not stable in Na2SO4 solution.245
Fabrication of a composite containing FeOOH and rGO was recently developed to obtain a high-performance electrode with good rate-capability and stability.246 FeOOH nanorod/rGO composites prepared by a solution method had a high electrochemical capacitance of 165.5 F g−1 with an excellent recycling capability.247 Urea was employed to reduce and dope GO to N-doped graphene, and simultaneously hydrolyzed to fabricate metal hydroxide. Thus, FeOOH nanorods could be randomly dispersed on N-doped graphene sheets. Specific capacitance of N-doped graphene/FeOOH nanorods was improved to be 309 F g−1.248
6. Other transition metal hydroxides
Beside Ni-, Co-, and Fe-based hydroxides, other transition metal hydroxides including Mn(OH)2, Cu(OH)2, and Cd(OH)2 also have been reported as electrode materials of ECs.Octahedral Mn(OH)2 nanoparticles with a size range from 140 to 200 nm have been fabricated by a sonochemical irradiation method for energy storage applications. These exhibited a specific capacitance of 127 F g−1 at a current density of 0.5 mA cm−2 in the potential range from −0.1 to 0.8 V in 1 M Na2SO4 solution.249 Using spray coating, Mn(OH)2/multi-walled CNT composite thin films were prepared on flexible indium tin oxide/polyethylene terephthalate substrate. The capacitance increased with the weight ratio of KMnO4/CNTs up to 1.6. The highest specific capacitance obtained at a scan rate of 20 mV s−1 was 297.5 F g−1 for the composite thin film with weight ratio of KMnO4/CNTs of 1.2.250
Copper hydroxide thin films on glass and stainless steel substrates were prepared by a soft chemical synthesis route at room temperature. The room temperature chemical synthesis route allowed formation of nanograined and hydrophilic Cu(OH)2 thin films, which exhibited specific capacitance of 120 F g−1 in 1 M NaOH solution.251
A conductive additive-free and binder-less Cd(OH)2 nanowires electrode was prepared via direct growth of Cd(OH)2 nanowires onto a nickel foam current collector via a simple solution growth method. The as-prepared Cd(OH)2 nanowires electrode showed excellent pseudocapacitive performance with specific capacitance of 1164.8 F g−1 at 1 A g−1 in 6 M KOH solution. However, the precursor solutions were harmful to the environment.252
The hydroxide decorated graphene composites displayed improved performance over pure MnSn(OH)6 nanoparticles because the graphene sheets acted as conductive bridges improving ionic and electronic transport. The total specific capacitance depended strongly on the crystallinity of the MnSn(OH)6 nanoparticles, where materials with poor crystallinity showed maximum specific capacitance of 31.2 F g−1 (59.4 F g−1 based on the mass of MnSn(OH)6 nanoparticles) at a scan rate of 5 mV s−1.253
7. Hierarchical composite materials containing metal hydroxides
Porous carbon materials, transition-metal oxides or hydroxides are promising candidates as electrode materials of ECs, but each of these materials has its own advantages and disadvantages. To achieve a breakthrough in energy-storage and energy-conversion devices for capacitors, facile fabrication of multiphase composites as electrodes is crucial, because these components enhance reaction kinetics and reduce cost. In this section, hierarchically porous heterostructure composites containing metal hydroxides as the electrode material of ECs are reviewed briefly.Ion transfer and electron conduction are two main factors that determine electrochemical performance of electrode materials of ECs. The hybrid nanostructured electrodes with high specific surface area and porous configuration can lead to a large electrode/electrolyte contact area, short diffusion path to current carriers, and high electron conductivity in electrodes.254,255 Huang et al. fabricated and tested the electrochemical performance of supercapacitor electrodes consisting of Ni(OH)2 nanosheets coated onto NiCo2O4 nanosheets grown on carbon fiber paper current collectors. When the NiCo2O4 nanosheets were replaced by Co3O4 nanosheets, however, energy and power density, as well as the rate capability of the electrodes, were significantly reduced because of the lower conductivity of Co3O4 than NiCo2O4.256 Similar work also has been carried out by Xu et al.,257 where NiCo2O4@CoxNi1−x(OH)2 core–shell nanosheet arrays on Ni foam had maximum areal capacitance of 887.5 mF cm−2. The Co3O4/Ni(OH)2 composite mesoporous nanosheet networks were synthesized on a conductive substrate for supercapacitor application by heat treatment of Co(OH)2/Ni(OH)2. The resulting products have been directly employed as EC electrodes, and exhibited predominant electrochemical performances, such as a high specific capacitance value of 1144 F g−1 at 5 mV s−1 and long-term cyclability.258
The core–shell nanostructures have shown promise in these systems recently, owing to the synergistic effects of the individual components, where the core materials generally have a high electron conductivity and the shells are porous oxides or hydroxides. A hybrid nanostructure of porous cobalt monoxide nanowire@ultrathin Ni(OH)2 nanoflake core–shell was directly synthesized on nickel foam by a two-step hydrothermal route, which demonstrated a specific capacitance of 798.3 F g−1 at a current density of 1.67 A g−1 and good rate performance as electrode material for supercapacitors, as reported by Guan.259 Nanoarchitectured fibrous Co3O4@Ni(OH)2 core–shell material grown on a nickel foam collector with excellent pseudocapacitive behaviors, was fabricated by combining hydrothermal synthesis and chemical-bath deposition methods. By combining the Co3O4@Ni(OH)2-based electrode with reduced GO or active carbon, a series of Co3O4@Ni(OH)2-based asymmetric EC prototypes were developed. These asymmetric ECs exhibited superior performance, such as high specific capacitance and high energy density. Because of the large mass loading and high energy density, the prototype could drive a minifan or light a bulb despite its very small size,260 as shown in Fig. 22. Co(OH)2 and Mn(OH)2 nanosheets also can be electrochemically deposited on the Co3O4 core nanowires to form core–shell arrays. The Co3O4/Co(OH)2 core–shell nanowire arrays were evaluated as a supercapacitor cathode material, which exhibited high specific capacitances of 1095 F g−1 at 1 A g−1 and 812 F g−1 at 40 A g−1, respectively.261 Co3O4@NiAl-LDH core–shell nanowire arrays with hierarchical structure have been synthesized by in situ growth of a LDH nanosheets shell on the surface of Co3O4 nanowire arrays, as reported by Duan group.262 This structure exhibited promising supercapacitance performance with largely enhanced specific capacitance and rate capability, much superior to pristine Co3O4 nanowire arrays because of its hierarchically mesoporous morphology and the strong core–shell binding interaction.
 | ||
| Fig. 22 (a) Schematic illustration of the fabricated Co3O4@Ni(OH)2//RGO asymmetric supercapacitor prototype in 6 M KOH electrolyte. Photographs of the Co3O4@Ni(OH)2//RGO asymmetric supercapacitor prototype as a power supply for a minifan (b) and a bulb (c). Reprinted with permission from ref. 260. Copyright (2013) American Chemical Society. | ||
In addition to these highly conductive metal oxides as the core of metal hydroxide, TiN nanowires were also applied as supports of Ni(OH)2, because TiN has great promise as an electrode material owing to its desirable electrical conductivity and mechanical stability.263 TiO2 nanorods were also selected as supports to combine with a Co(OH)2 nanowall array, thus a novel hierarchical electrode with improved performance was obtained.264 Even K2Ti4O9 nanowires grown on Ti substrate were coated by Ni(OH)2 nanosheets to make a core–shell heterostructure, where the heterostructure delivered high specific capacitance in aqueous and solid-state electrolytes.265
Sun et al. designed and synthesized 3D hierarchical heterostructures of dense MnOOH nanosheets on porous hierarchical NiO nanosheet arrays, as shown in Fig. 23. In this configuration, porous hierarchical NiO nanosheet arrays serve as a fast ion and electron transport model, and MnOOH ultrathin nanosheets can enhance the contact surface area and assist ion penetration into the core region to realize release of potential electrochemical properties of NiO nanosheet arrays. These heterostructures provide intense necessary critical function for efficient use of metal oxide and hydroxide in ECs. As an electrode, the 3D NiO@MnOOH core–shell nanosheet hierarchies exhibited excellent electrochemical performances, i.e. high specific capacitance of 1625.3 F g−1 at a current density of 4 A g−1 with good rate capability and high energy density (80.0 W h kg−1).266
 | ||
| Fig. 23 (a and b) SEM images of the NiO@MnOOH core–shell nanosheet hierarchies. (c and d) TEM and HRTEM images of the MnOOH nanosheets, two lower-right insets in (c and d) are the corresponding SAED and FFT patterns, respectively. Reprinted with permission from ref. 266. Copyright (2014) Elsevier Ltd. | ||
Very recently, Kang et al. reported an approach for fabricating low-cost transition-metal based oxy-hydroxide@nanoporous metal electrodes by electrochemical polarization of a dealloyed nanoporous Ni–Mn alloy in an alkaline solution. The hybrid electrode had a high volumetric specific capacitance (505 F cm−3) and excellent rate-capacity performance.267 This study may pave a new way for fabricating oxide-hydroxide/nanoporous metal hybrid electrodes with unprecedented properties for high-performance ECs.
8. Asymmetric supercapacitors based on metal hydroxide
According to the cell configuration for ECs, there are two kinds of ECs, symmetric and asymmetric/or hybrid ECs. Symmetric ECs are formed with two similar electrode materials as cathode and anode, whereas two dissimilar electrode materials form asymmetric ECs. The asymmetric design is an attractive approach to increasing the energy density of ECs as it can lead to an almost doubling of device capacitance. Asymmetric ECs incorporate both a polarizable and non-polarizable electrode, consisting of a polarizable electrode (usually high surface area carbon) and a battery-type electrode (usually a Faradic or intercalating metal oxide).268−270 It is difficult to have a higher capacitance with higher operating voltage in common symmetric ECs because of the limited potential window, whereas asymmetric capacitors can deliver charge with a higher operating voltage.271 Asymmetric or hybrid ECs are regarded as the new trend in supercapacitors.272Co(OH)2 is an important electrode material for asymmetric supercapacitors, and there have been several reports on its application in asymmetric ECs.273,274 Asymmetric capacitor CNT-α-Co(OH)2 was prepared with a low electrode resistance of 0.42 Ω cm2. Although α-Co(OH)2 raises the electrode resistance, it enhances the cell energy capacity greatly to 7.8 W h kg−1.275 Cheng et al. fabricated graphene–CNT and graphene–CNT–Co(OH)2 electrodes and assembled them in asymmetric ECs. Single-walled CNTs could act as a conductive spacer as well as a conductive binder in the composite. A high energy density of 172 W h kg−1 and a maximum power density of 198 kW kg−1 were obtained in ionic liquid electrolyte EMI-TFSI.276
Use of nickel hydroxide or its composites is also popular as positive electrodes of asymmetric ECs with high specific capacitance and high energy density,277 typically for hydroxide films anchored directly on a current collector.278−281 The asymmetric EC with the highest power density of 44 W h kg−1 was made using porous β-Ni(OH)2 films deposited on lightweight and highly conductive surface ultrathin-graphite foam as the positive electrode, and microwave exfoliated GO as negative electrode, as shown in Fig. 24. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, 63.2% capacitance remained. Its highest power density is comparable with or higher than that of high-end commercially available supercapacitors.279 An asymmetric EC consisting of Ni(OH)2/CNTs directly grown on Ni foam positive electrode and active carbon negative electrode, can deliver cell voltage of 1.8 V and an energy density of 50.6 W h kg−1, about 10 times higher than that of a traditional electrochemical double layer capacitor, as reported by Gao.280 The graphene-supported Ni(OH)2-nanowires and CMK-5 were used as the positive and negative electrodes, respectively, to form a high performance hybrid supercapacitor which could deliver a maximum specific power density of 40
000 cycles, 63.2% capacitance remained. Its highest power density is comparable with or higher than that of high-end commercially available supercapacitors.279 An asymmetric EC consisting of Ni(OH)2/CNTs directly grown on Ni foam positive electrode and active carbon negative electrode, can deliver cell voltage of 1.8 V and an energy density of 50.6 W h kg−1, about 10 times higher than that of a traditional electrochemical double layer capacitor, as reported by Gao.280 The graphene-supported Ni(OH)2-nanowires and CMK-5 were used as the positive and negative electrodes, respectively, to form a high performance hybrid supercapacitor which could deliver a maximum specific power density of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 W kg−1 with a high energy density of about 17.3 W h kg−1.282 Yan et al. assembled an asymmetric EC using α-Ni(OH)2/graphene and porous graphene as the positive and negative electrodes, respectively. The asymmetrical EC showed a high specific capacitance of 218.4 F g−1, high energy density (77.8 W h kg−1), and good cycling stability at an operating voltage of about 1.6 V in KOH aqueous electrolytes.283 Wang et al. compared the asymmetric ECs made of Ni(OH)2/graphene with RuO2/graphene. The ECs made of Ni(OH)2/graphene and RuO2/graphene showed high specific capacitance and a high energy density with a 1.5 V operating voltage.284
840 W kg−1 with a high energy density of about 17.3 W h kg−1.282 Yan et al. assembled an asymmetric EC using α-Ni(OH)2/graphene and porous graphene as the positive and negative electrodes, respectively. The asymmetrical EC showed a high specific capacitance of 218.4 F g−1, high energy density (77.8 W h kg−1), and good cycling stability at an operating voltage of about 1.6 V in KOH aqueous electrolytes.283 Wang et al. compared the asymmetric ECs made of Ni(OH)2/graphene with RuO2/graphene. The ECs made of Ni(OH)2/graphene and RuO2/graphene showed high specific capacitance and a high energy density with a 1.5 V operating voltage.284
 | ||
| Fig. 24 SEM images of the Ni(OH)2/ultrathin graphite foam composite, (a) SEM image of the composite material, and the inset shows a higher magnification image of the Ni(OH)2 nanoflakes; (b) a cross-sectional view of the composite; (c) SEM image and the corresponding EDS elemental mapping images of (d) carbon, (e) oxygen, and (f) nickel. Reprinted with permission from ref. 279. Copyright (2013) American Chemical Society. | ||
Guest metal doping or substitution in Ni(OH)2 was reported to improve the electrode materials of asymmetric ECs. The Kong group reported that Al-substitution could make a stabilized α-Ni(OH)2 and 7.5% Al containing α-Ni(OH)2 exhibited a specific capacitance of 127 F g−1 and energy density of 42 W h kg−1.285 The XRD results proved that addition of Al over 7.5% led to higher crystallinity and increased the structure stability in alkaline medium. Ni(OH)2 prepared by co-precipitation with Zn and Co as positive electrode material of an asymmetric EC, both exhibited a higher specific capacitance than pure Ni(OH)2.286 The improved properties were attributed to co-precipitated Zn and Co, because the formed Zn(OH)2 with Ni(OH)2 could inhibit formation of γ-NiOOH and the distributed cobalt in the lattice or on the surface of Ni(OH)2 could maintain good conductivity.286
Meanwhile, some LDHs such as NiAl-,287 CoAl-,288 and CoNi-287,289−293 LDHs or their composites, and Ni(OH)256,294−296 also have been fabricated as electrodes of asymmetric supercapacitors. They all showed great potential energy storage ability and high rate capability.
9. Conclusion and prospects
Earth-abundant metal hydroxides as electrode materials are suitable for development of ECs. These low-cost and high-performance active hydroxide materials realized by simple and scalable solution processes offer great promise in development of novel materials for future energy storage devices. An effective route is use of 3D porously hierarchical hydroxides. These 3D pseduocapacitor electrodes have a number of features, such as fast ion and electron transfer, easy access of pseudoactive species, and efficient utilization and excellent reversibility. Although 3D nanoporous architectures have been shown to have advantages in supercapacitors, design and fabrication of high performance electrode materials with 3D hierarchical structures with large specific area and excellent rate capability remains a challenge.Although most transition metal hydroxides, such as Co(OH)2, Ni(OH)2, and CoAl-, NiAl-LDHs are promising electrode materials for electrochemical capacitors on account of their high theoretical specific capacitance and low cost, application is hindered by their low measured specific capacitance and poor cycle stability, which are often associated with low specific surface area and poor electrical conductivity. There are two methods that can be applied to overcome these problems. One method is combining the metal hydroxide into a composite with an electronically conductive material such as carbon nanotube, graphene (reduced GO), and active carbon. The conductive material acts as both support and electron collector, while the hydroxide plays the role of electrochemically active species to form a synergistic effect. Composites consisting of metal hydroxides and carbon materials are promising for EC applications. The other method is direct fabrication of metal hydroxide crystal films onto a current collector. These films of transition metal hydroxides deposited on conductive substrates can be used directly as EC electrodes (polymer binders and conducting agents are not required), with high specific capacitance and good rate capability. Therefore, various approaches have been employed to prepare nanostructure films of metal hydroxides on conductive substrates directly, typically for hydrothermal and electrochemical deposition. However, these two methods have some intrinsic shortcomings. The hydrothermal method is limited by volume size, in continuous procedure, and long reaction time. The electrochemical deposition technique has disadvantages including small area of deposition, extreme cleaning after each deposition, and high working cost. Moreover, the mass loading of active materials on the substrate is usually low and difficult to control precisely. Thus, these issues limit application of hydroxide films as electrodes on a large scale.
For practical application, cyclic stability is crucial for an electrode material in electrochemical capacitors. Excellent cycle performance can be attributed to a few factors. One of the most important factors is the stable structure of electrode material. The structure and phase of the electroactive materials should not change during repeated charge–discharge cycles. Typically for these porous materials, pore size and volume are of great significance for good ion diffusion in the electrolyte to the electrode surface. Some reports found that capacitance decay was caused by dissolution of active materials in the electrolyte. Facile electron transport through the electrode material to the current collector should be guaranteed, facilitating electrons to the current collector to improve electronic and ionic conductivities. Thus, low internal resistance of the electrode is necessary. Good wettability/or accessibility between electrolyte and electrode materials is also needed, therefore, consideration should be made of the functional groups on electrode materials.
It is clear that the structure of metal hydroxides has great influence on their electrochemical properties. Hydrotalcite-like structured hydroxides show greater potential than hydroxide materials with a brucite structure. Owing to their tunable composition, and flexible interlayer spacing, hydrotalcite-like hydroxides have been deemed one of the most outstanding electrode materials for ECs, including α-Co(OH)2 and α-Ni(OH)2. However, the susceptibility of their structure in an alkaline media as electrolyte is an inevitable disadvantage.
From the theoretical viewpoint, hierarchical heterostructure composites containing metal hydroxides as the shell and semiconducting metal oxide as the core, are promising as electrode materials of ECs. The hybrid nanostructured electrodes usually have a high specific surface area and porous configuration, leading to a large electrode/electrolyte contact area, short diffusion path to current carriers, and high electron conductivity. However, the fabrication processes for these heterostructure composites are multi-stepped and complicated, a large obstacle to their practical application in EC electrodes.
Acknowledgements
This work was financially supported by the Zhejiang Provincial Natural Science Foundation of China under grant no. LY13E020002, Experimental research project of Zhejiang University.References
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- X. Li and B. Q. Wei, Nano Energy, 2013, 2, 159–173 CrossRef CAS PubMed.
- (a) J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed; (b) K. Wang, H. P. Wu, Y. N. Meng and Z. X. Wei, Small, 2014, 10, 14–31 CrossRef CAS PubMed.
- G. P. Wang, L. Zhang and J. J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- S. Faraji and F. N. Ani, J. Power Sources, 2014, 263, 338–360 CrossRef CAS PubMed.
- Q. Yang, Z. Y. Lu, J. F. Liu, X. D. Lei, L. Luo and X. M. Sun, Prog. Nat. Sci. Mater. Int., 2013, 23, 351–366 CrossRef PubMed.
- X. Peng, L. L. Peng, C. Z. Wu and Y. Xie, Chem. Soc. Rev., 2014, 43, 3303–3323 RSC.
- S. Liu, S. H. Sun and X. Z. You, Nanoscale, 2014, 6, 2037–2045 RSC.
- Z. S. Wu, G. M. Zhou, L. C. Yin, W. C. Ren, F. Li and H. M. Cheng, Nano Energy, 2012, 1, 107–131 CrossRef CAS PubMed.
- C. D. Lokhande, D. P. Dubal and O. S. Joo, Curr. Appl. Phys., 2011, 11, 255–270 CrossRef PubMed.
- W. T. Deng, X. B. Ji, Q. Y. Chen and C. E. Banks, RSC Adv., 2011, 1, 1171–1178 RSC.
- W. F. Wei, X. W. Cui, W. X. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- C. J. Xu, F. Y. Kang, B. H. Li and H. D. Du, J. Mater. Res., 2010, 25, 1421–1432 CrossRef CAS.
- M. Aghazadeh, S. Dalvand and M. Hosseinifard, Ceram. Int., 2014, 40, 3485–3493 CrossRef CAS PubMed.
- X. H. Liu, B. H. Qu, F. H. Zhu, L. Y. Gong, L. H. Su and L. Q. Zhu, J. Alloys Compd., 2013, 560, 15–19 CrossRef CAS PubMed.
- M. Aghazadeh, H. M. Shiri and A. A. M. Barmi, Appl. Surf. Sci., 2013, 273, 237–242 CrossRef CAS PubMed.
- (a) L. Cao, F. Xu, Y. Y. Liang and H. L. Li, Adv. Mater., 2014, 16, 1853–1857 CrossRef PubMed; (b) Z. A. Hu, L. P. Mo, X. J. Feng, J. Shi, Y. X. Wang and Y. L. Xie, Mater. Chem. Phys., 2009, 114, 53–57 CrossRef CAS PubMed.
- J. P. Cheng, M. Li, W. F. Zhang, J. S. Wu, F. Liu and X. B. Zhang, RSC Adv., 2013, 3, 13304–13310 RSC.
- L. Y. Gong and L. H. Su, Appl. Surf. Sci., 2011, 257, 10201–10205 CrossRef CAS PubMed.
- X. B. Ji, P. M. Hallam, S. M. Houssein, R. Kadara, L. M. Lang and C. E. Banks, RSC Adv., 2012, 2, 1508–1515 RSC.
- Y. F. Tang, Y. Y. Liu, S. X. Yu, S. C. Mu, S. H. Xiao, Y. F. Zhao and F. M. Gao, J. Power Sources, 2014, 256, 160–169 CrossRef CAS PubMed.
- M. Aghazadeh and S. Dalvand, J. Electrochem. Soc., 2014, 161, 18–25 CrossRef PubMed.
- S. C. Tang, S. Vongehr, Y. Wang, L. Chen and X. K. Meng, J. Solid State Chem., 2010, 183, 2166–2173 CrossRef CAS PubMed.
- J. P. Cheng, X. Chen, J. S. Wu, F. Liu, X. B. Zhang and V. P. Dravid, CrystEngComm, 2012, 14, 6702–6709 RSC.
- X. Chen, J. P. Cheng, Q. L. Shou, F. Liu and X. B. Zhang, CrystEngComm, 2012, 14, 1271–1276 RSC.
- L. B. Kong, J. J. Cai, L. L. Sun, J. Zhang, Y. C. Luo and L. Kong, Mater. Chem. Phys., 2010, 122, 368–373 CrossRef CAS PubMed.
- C. Mondal, M. Ganguly, P. K. Manna, S. M. Yusuf and T. Pal, Langmuir, 2013, 29, 9179–9187 CrossRef CAS PubMed.
- C. M. Wu, C. Y. Fan, I. W. Sun, W. T. Tsai and J. K. Chang, J. Power Sources, 2011, 196, 7828–7834 CrossRef CAS PubMed.
- J. K. Chang, C. M. Wu and I. W. Sun, J. Mater. Chem., 2010, 20, 3729–3735 RSC.
- C. Y. Men, Q. Shi, J. Li and Q. W. Li, J. Inorg. Mater., 2013, 28, 1321–1327 CrossRef CAS.
- X. M. Ni, H. G. Zheng, X. K. Xiao, X. Jin and G. X. Liao, J. Alloys Compd., 2009, 484, 467–471 CrossRef CAS PubMed.
- T. Xue and J. M. Lee, J. Power Sources, 2014, 245, 194–202 CrossRef CAS PubMed.
- T. Xue, X. Wang and J. M. Lee, J. Power Sources, 2012, 201, 382–386 CrossRef CAS PubMed.
- C. Z. Yuan, X. G. Zhang, L. R. Hou, L. F. Shen, D. K. Li, F. Zhang, C. G. Fan and J. M. Li, J. Mater. Chem., 2010, 20, 10809–10816 RSC.
- X. Tian, H. Y. Gao, M. Yang, Y. Luan, W. J. Dong, Z. K. Jin, J. Yu, Y. Qi, Y. H. Feng and G. Wang, J. Alloys Compd., 2014, 608, 278–282 CrossRef PubMed.
- A. D. Jagadale, V. S. Jamadade, S. N. Pusawale and C. D. Lokhande, Electrochim. Acta, 2012, 78, 92–97 CrossRef CAS PubMed.
- W. J. Zhou, D. D. Zhao, M. W. Xu, C. L. Xu and H. L. Li, Electrochim. Acta, 2008, 53, 7210–7219 CrossRef CAS PubMed.
- M. Li, S. H. Xu, T. Liu, F. Wang, P. X. Yang, L. W. Wang and P. K. Chu, J. Mater. Chem. A, 2013, 1, 532–540 CAS.
- W. J. Zhou, M. W. Xu, D. D. Zhao, C. L. Xu and H. L. Li, Microporous Mesoporous Mater., 2009, 117, 55–60 CrossRef CAS PubMed.
- W. J. Zhou, J. Zhang, T. Xue, D. D. Zhao and H. L. Li, J. Mater. Chem., 2008, 18, 905–910 RSC.
- A. D. Jagadale, V. S. Kumbhar, D. S. Dhawale and C. D. Lokhande, Electrochim. Acta, 2013, 98, 32–38 CrossRef CAS PubMed.
- A. A. M. Barmi, M. Aghazadeh, B. Arhami, H. M. Shiri, A. A. Fazl and E. Jangju, Chem. Phys. Lett., 2012, 541, 65–69 CrossRef PubMed.
- F. Cao, G. X. Pan, P. S. Tang and H. F. Chen, J. Power Sources, 2012, 216, 395–399 CrossRef CAS PubMed.
- B. G. Choi, M. H. Yang, S. C. Jung, K. G. Lee, J. G. Kim, H. S. Park, T. J. Park, S. B. Lee, Y. K. Han and Y. S. Huh, ACS Nano, 2013, 7, 2453–2460 CrossRef CAS PubMed.
- Z. X. Tai, J. W. Lang, X. B. Yan and Q. J. Xue, J. Electrochem. Soc., 2012, 159, 485–491 CrossRef PubMed.
- R. R. Salunkhe, B. P. Bastakoti, C. T. Hsu, N. Suzuki, J. H. Kim, S. X. Dou, C. C. Hu and Y. Yamauchi, Chem.–Eur. J., 2014, 20, 3084–3088 CrossRef CAS PubMed.
- L. Wang, C. Lin, F. X. Zhang and J. Jin, ACS Nano, 2014, 8, 3724–3734 CrossRef CAS PubMed.
- A. K. Mondal, D. Su, S. Q. Chen, B. Sun, K. F. Li and G. X. Wang, RSC Adv., 2014, 4, 19476–19481 RSC.
- G. J. Shao, Y. Yao, S. P. Zhang and P. He, Rare Met., 2009, 28, 132–136 CrossRef CAS PubMed.
- J. Li, F. L. Luo, X. Q. Tian, Y. Lei, H. Y. Yuan and D. Xiao, J. Power Sources, 2013, 243, 721–727 CrossRef CAS PubMed.
- J. W. Lang, L. B. Kong, W. J. Wu, M. Liu, Y. C. Luo and L. Kang, J. Solid State Electrochem., 2009, 13, 333–340 CrossRef CAS.
- Y. Tian, X. H. Zhou, L. P. Huang and M. Liu, J. Inorg. Organomet. Polym., 2013, 23, 1425–1430 CrossRef CAS.
- S. T. Xing, Q. Wang, Z. C. Ma, Y. S. Wu and Y. Z. Gao, Ionics, 2013, 19, 651–656 CrossRef CAS.
- H. L. Li, S. Q. Liu, C. H. Huang, Z. Zhou, Y. H. Li and D. Fang, Electrochim. Acta, 2011, 58, 89–94 CrossRef CAS PubMed.
- X. M. Ni, H. G. Zheng, X. K. Xiao, X. Jin and G. X. Liao, J. Alloys Compd., 2009, 484, 467–471 CrossRef CAS PubMed.
- Y. F. Tang, Y. Y. Liu, S. X. Yu, Y. F. Zhao, S. C. Mu and F. M. Gao, Electrochim. Acta, 2014, 123, 158–166 CrossRef CAS PubMed.
- Y. Wang, S. L. Gai, C. X. Li, F. He, M. L. Zhang, Y. D. Yan and P. P. Yang, Electrochim. Acta, 2013, 90, 673–681 CrossRef CAS PubMed.
- M. S. Wu and K. C. Huang, Chem. Commun., 2011, 47, 12122–12124 RSC.
- H. J. Yan, J. Wang, S. N. Li, W. L. Yang, Z. Gao, Q. Liu, R. Gao, Z. S. Li, M. L. Zhang and L. H. Liu, Electrochim. Acta, 2013, 87, 880–888 CrossRef CAS PubMed.
- Z. J. Luo, K. Wang, H. M. Li, S. Yin, Q. F. Guan and L. G. Wang, CrystEngComm, 2011, 13, 7108–7113 RSC.
- M. S. Wu and J. F. Wu, J. Power Sources, 2013, 240, 397–403 CrossRef CAS PubMed.
- M. Wang, Y. H. Ni, L. Cao, D. Zhao and X. Ma, J. Colloid Interface Sci., 2013, 401, 8–13 CrossRef CAS PubMed.
- Z. Y. Lu, Z. Chang, W. Zhu and X. M. Sun, Chem. Commun., 2011, 47, 9651–9653 RSC.
- G. W. Yang, C. L. Xu and H. L. Li, Chem. Commun., 2008, 6537–6539 RSC.
- Y. M. Wang, D. D. Zhao, Y. Q. Zhao, C. L. Xu and H. L. Li, RSC Adv., 2012, 2, 1074–1082 RSC.
- D. S. Kong, J. M. Wang, H. B. Shao, J. Q. Zhang and C. N. Cao, J. Alloys Compd., 2011, 509, 5611–5616 CrossRef CAS PubMed.
- Y. H. Ko, S. Kim and J. S. Yu, Mater. Lett., 2012, 84, 132–135 CrossRef CAS PubMed.
- Y. F. Yuan, X. H. Xia, J. B. Wu, J. L. Yang, Y. B. Chen and S. Y. Guo, Electrochim. Acta, 2011, 56, 2627–2632 CrossRef CAS PubMed.
- M. Aghazadeh, A. N. Golikand and M. Ghaemi, Int. J. Hydrogen Energy, 2011, 36, 8674–8679 CrossRef CAS PubMed.
- D. P. Dubal, V. J. Fulari and C. D. Lokhande, Microporous Mesoporous Mater., 2012, 151, 511–516 CrossRef CAS PubMed.
- D. P. Dubal, S. H. Lee and W. B. Kim, J. Mater. Sci., 2012, 47, 3817–3821 CrossRef CAS.
- X. B. Zhou, D. X. Cao, J. C. Huang, K. Ye, S. N. Yang, T. Liu, X. W. Liu, J. L. Yin and G. L. Wang, J. Electroanal. Chem., 2014, 720–721, 115–120 CrossRef CAS PubMed.
- B. L. Hu, X. Y. Qin, A. M. Asiri and K. A. Alamry, Electrochim. Acta, 2013, 107, 339–342 CrossRef CAS PubMed.
- S. Lv, H. Suo, J. M. Wang, Y. Wang, C. Zhao and S. X. Xing, Colloids Surf., A, 2012, 396, 292–298 CrossRef CAS PubMed.
- L. C. Wu, Y. J. Chen, M. L. Mao, Q. H. Li and M. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 5168–5174 CAS.
- G. S. Gund, D. P. Dubal, S. S. Shinde and C. D. Lokhande, Ceram. Int., 2013, 39, 7255–7261 CrossRef CAS PubMed.
- J. Q. Tian, Z. C. Xing, Q. X. Chu, Q. Liu, A. M. Asiri, A. H. Qusti, A. O. Al-Youbi and X. P. Sun, CrystEngComm, 2013, 15, 8300–8305 RSC.
- G. S. Gund, D. P. Dubal, S. B. Jambure, S. S. Shinde and C. D. Lokhande, J. Mater. Chem. A, 2013, 1, 4793–4803 CAS.
- (a) H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed; (b) Y. Fu, J. M. Song, Y. Q. Zhu and C. B. Cao, J. Power Sources, 2014, 262, 344–348 CrossRef CAS PubMed.
- H. B. Li, M. H. Yu, X. H. Lu, P. Liu, Y. Liang, J. Xiao, Y. X. Tong and G. W. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 745–749 CAS.
- J. Zhang, L. B. Kong, J. J. Cai, Y. C. Luo and L. Kang, J. Solid State Electrochem., 2010, 14, 2065–2075 CrossRef CAS PubMed.
- C. Y. Sun, Y. G. Zhu, T. J. Zhu, J. Xie, G. S. Cao and X. B. Zhao, J. Solid State Electrochem., 2013, 17, 1159–1165 CrossRef CAS PubMed.
- (a) D. Ghosh, S. Giri and C. K. Das, ACS Sustainable Chem. Eng., 2013, 1, 1135–1142 CrossRef CAS; (b) W. F. Zhang, F. Liu, Q. Q. Li, Q. L. Shou, J. P. Cheng, L. Zhang, B. J. Nelson and X. B. Zhang, Phys. Chem. Chem. Phys., 2014, 14, 16331–16337 RSC.
- S. Chen, J. W. Zhu and X. Wang, J. Phys. Chem. C, 2010, 114, 11829–11834 CAS.
- Z. P. Li, J. P. Wang, L. Y. Niu, J. F. Sun, P. W. Gong, W. Hong, L. M. Ma and S. R. Yang, J. Power Sources, 2014, 245, 224–231 CrossRef CAS PubMed.
- (a) Y. Wang, S. L. Gai, N. Niu, F. He and P. P. Yang, J. Mater. Chem. A, 2013, 1, 9083–9091 RSC; (b) H. X. Chang, J. L. Kang, L. Y. Chen, J. Q. Wang, K. Ohmura, N. Chen, T. Fujita, H. K. Wu and M. W. Chen, Nanoscale, 2014, 6, 5960–5966 RSC.
- H. J. Yan, J. W. Bai, J. Wang, X. Y. Zhang, B. Wang, Q. Liu and L. H. Liu, CrystEngComm, 2013, 15, 10007–10015 RSC.
- D. L. Fang, Z. D. Chen, X. Liu, Z. F. Wu and C. H. Zheng, Electrochim. Acta, 2012, 81, 321–329 CrossRef CAS PubMed.
- S. Chen, J. J. Duan, Y. H. Tang and S. Z. Qiao, Chem.–Eur. J., 2013, 19, 7118–7124 CrossRef CAS PubMed.
- Y. Y. He, J. J. Zhang and J. W. Zhao, Acta Phys.–Chim. Sin., 2014, 30, 297–304 CAS.
- J. F. Xie, X. Sun, N. Zhang, K. Xu, M. Zhao and Y. Xie, Nano Energy, 2013, 2, 65–74 CrossRef CAS PubMed.
- S. D. Min, C. J. Zhao, G. R. Chen and X. Z. Qian, Electrochim. Acta, 2014, 115, 155–164 CrossRef CAS PubMed.
- H. T. Zhang, X. Zhang, D. C. Zhang, X. Z. Sun, H. Lin, C. H. Wang and Y. W. Ma, J. Phys. Chem. B, 2013, 117, 1616–1627 CrossRef CAS PubMed.
- C. H. Jiang, B. B. Zhan, C. Li, W. Huang and X. C. Dong, RSC Adv., 2014, 4, 18080–18085 RSC.
- H. L. Wang, H. S. Casalongue, Y. Y. Liang and H. J. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed.
- Z. Wu, X. L. Huang, Z. L. Wang, J. J. Xu, H. G. Wang and X. B. Zhang, Sci. Rep., 2014, 4, 3669 Search PubMed.
- M. Shahid, J. L. Liu, I. Shakir, M. F. Warsi, M. Nadeem and Y. U. K. Kwon, Electrochim. Acta, 2012, 85, 243–247 CrossRef CAS PubMed.
- D. P. Dubal, G. S. Gund, C. D. Lokhande and R. Holze, ACS Appl. Mater. Interfaces, 2013, 5, 2446–2454 CAS.
- H. Chai, X. Chen, D. Z. Jia and W. Y. Zhou, Rare Met., 2011, 30, 85–89 CrossRef CAS.
- J. Zhang, L. B. Kong, J. J. Cai, H. Li, Y. C. Luo and L. Kang, Microporous Mesoporous Mater., 2010, 132, 154–162 CrossRef CAS PubMed.
- L. L. Zhang, Z. G. Xiong and X. S. Zhao, J. Power Sources, 2013, 222, 326–332 CrossRef CAS PubMed.
- Z. P. Sun and X. M. Lu, Ind. Eng. Chem. Res., 2012, 51, 9973–9979 CrossRef CAS.
- J. Han, K. C. Roh, M. R. Jo and Y. M. Kang, Chem. Commun., 2013, 49, 7067–7069 RSC.
- H. Chen, L. F. Hu, M. Chen, Y. Yan and L. M. Wu, Adv. Funct. Mater., 2014, 24, 934–942 CrossRef CAS PubMed.
- H. Chen, L. F. Hu, Y. Yan, R. C. Che, M. Chen and L. M. Wu, Adv. Energy Mater., 2013, 3, 1636–1646 CrossRef CAS PubMed.
- X. H. Liu, R. Z. Ma, Y. Bando and T. Sasaki, Adv. Mater., 2012, 24, 2148–2153 CrossRef CAS PubMed.
- X. Wang, A. Sumboja, M. F. Lin, J. Yan and P. S. Lee, Nanoscale, 2012, 4, 7266–7272 RSC.
- N. T. H. Trang, H. V. Ngoc, N. Lingappan and D. H. Kang, Nanoscale, 2014, 6, 2434–2439 RSC.
- R. R. Salunkhe, K. Jang, S. W. Lee and H. Ahn, RSC Adv., 2012, 2, 3190–3193 RSC.
- L. J. Xie, Z. G. Hu, C. X. Lv, G. H. Sun, J. L. Wang, Y. Q. Li, H. W. He, J. Wang and K. X. Li, Electrochim. Acta, 2012, 78, 205–211 CrossRef CAS PubMed.
- Z. Jiang, Z. P. Li, Z. H. Qin, H. Y. Sun, X. L. Jiao and D. R. Chen, Nanoscale, 2013, 5, 11770–11775 RSC.
- T. Yan, H. Y. Zhu, R. Y. Li, Z. J. Li, J. K. Liu, G. L. Wang and Z. Q. Gu, Electrochim. Acta, 2013, 111, 71–79 CrossRef PubMed.
- T. Yan, Z. J. Li, R. Y. Li, Q. Ning, H. Kong, Y. L. Niu and J. K. Liu, J. Mater. Chem., 2012, 22, 23587–23592 RSC.
- V. Gupta, S. Gupta and N. Miura, J. Power Sources, 2008, 175, 680–685 CrossRef CAS PubMed.
- Z. A. Hu, Y. L. Xie, Y. X. Wang, H. Y. Wu, Y. Y. Yang and Z. Y. Zhang, Electrochim. Acta, 2009, 54, 2737–2741 CrossRef CAS PubMed.
- S. B. Kulkarni, A. D. Jagadale, V. S. Kumbhar, R. N. Bulakhe, S. S. Joshi and C. D. Lokhande, Int. J. Hydrogen Energy, 2013, 38, 4046–4053 CrossRef CAS PubMed.
- T. Yan, R. Y. Li, Z. J. Li, J. K. Liu, G. L. Wang and Z. Q. Gu, RSC Adv., 2013, 3, 19416–19422 RSC.
- J. C. Chen, C. T. Hsu and C. C. Hu, J. Power Sources, 2014, 253, 205–213 CrossRef CAS PubMed.
- J. Pu, Y. Tong, S. B. Wang, E. H. Sheng and Z. H. Wang, J. Power Sources, 2014, 250, 250–256 CrossRef CAS PubMed.
- I. Shakir, M. Shahid, U. A. Rana, I. M. A. Nashef and R. Hussain, Electrochim. Acta, 2014, 129, 28–32 CrossRef CAS PubMed.
- M. F. Warsi, I. Shakir, M. Shahid, M. Sarfraz, M. Nadeem and Z. A. Gilani, Electrochim. Acta, 2014, 135, 513–518 CrossRef CAS PubMed.
- J. Xu, Y. Z. Dong, J. Y. Cao, B. Guo, W. C. Wang and Z. D. Chen, Electrochim. Acta, 2013, 114, 76–82 CrossRef CAS PubMed.
- M. Sebastian, C. Nethravathi and M. Rajamathi, Mater. Res. Bull., 2013, 48, 2715–2719 CrossRef CAS PubMed.
- X. Sun, G. K. Wang, H. T. Sun, F. Y. Lu, M. P. Yu and J. Lian, J. Power Sources, 2013, 238, 150–156 CrossRef CAS PubMed.
- G. Chen, S. S. Liaw, B. S. Li, Y. Xu, M. Dunwell and S. G. Deng, J. Power Sources, 2013, 251, 338–343 CrossRef PubMed.
- Y. W. Cheng, H. B. Zhang, C. V. Varanasi and J. Liu, Energy Environ. Sci., 2013, 6, 3314–3321 CAS.
- T. Yan, R. Y. Li and Z. J. Li, Mater. Res. Bull., 2014, 51, 97–104 CrossRef CAS PubMed.
- L. Y. Zhang, C. H. Tang, X. S. Yin and H. Gong, J. Mater. Chem. A, 2014, 2, 4660–4666 CAS.
- X. M. Liu, Y. H. Zhang, X. G. Zhang and S. Y. Fu, Electrochim. Acta, 2004, 49, 3137–3141 CrossRef CAS PubMed.
- P. Vialat, F. Leroux, C. T. Gueho, G. Villemure and C. Mousty, Electrochim. Acta, 2013, 107, 599–610 CrossRef CAS PubMed.
- Z. Y. Lu, W. Zhu, X. D. Lei, G. R. Williams, D. O. Hare, Z. Chang, X. M. Sun and X. Duan, Nanoscale, 2012, 4, 3640–3643 RSC.
- W. Hong, J. Q. Wang, L. Y. Niu, J. F. Sun, P. W. Gong and S. R. Yang, J. Alloys Compd., 2014, 608, 297–303 CrossRef CAS PubMed.
- Y. Wang, W. S. Yang, C. Chen and D. G. Evans, J. Power Sources, 2008, 184, 682–690 CrossRef CAS PubMed.
- L. Wang, D. Wang, X. Y. Dong, Z. J. Zhang, X. F. Pei, X. J. Chen, B. Chen and J. Jin, Chem. Commun., 2011, 47, 3556–3558 RSC.
- X. Y. Dong, L. Wang, D. Wang, C. Li and J. Jin, Langmuir, 2012, 28, 293–298 CrossRef CAS PubMed.
- M. Hu, X. D. Ji, L. X. Lei and X. W. Lu, Electrochim. Acta, 2013, 105, 261–274 CrossRef CAS PubMed.
- J. P. Cheng, J. H. Fang, M. Li, W. F. Zhang, F. Liu and X. B. Zhang, Electrochim. Acta, 2013, 114, 68–75 CrossRef CAS PubMed.
- J. B. Han, Y. B. Dou, J. W. Zhao, M. Wei, D. G. Evans and X. Duan, Small, 2013, 9, 98–106 CrossRef CAS PubMed.
- L. J. Zhang, X. G. Zhang, L. F. Shen, B. Gao, L. Hao, X. J. Lu, F. Zhang, B. Ding and C. Z. Yuan, J. Power Sources, 2012, 199, 395–401 CrossRef CAS PubMed.
- S. Huang, G. N. Zhu, C. Zhang, W. W. Tjiu, Y. Y. Xia and T. X. Liu, ACS Appl. Mater. Interfaces, 2012, 4, 2242–2249 CAS.
- J. H. Hao, W. S. Yang, Z. Zhang, B. P. Lu, X. Ke, B. L. Zhang and J. L. Tang, J. Colloid Interface Sci., 2014, 426, 131–136 CrossRef CAS PubMed.
- J. H. Fang, M. Li, Q. Q. Li, W. F. Zhang, Q. L. Shou, F. Liu, X. B. Zhang and J. P. Cheng, Electrochim. Acta, 2012, 85, 248–255 CrossRef CAS PubMed.
- A. Faour, C. Mousty, V. Prevot, B. Devouard, A. D. Roy, P. Bordet, E. Elkaim and C. T. Gueho, J. Phys. Chem. C, 2012, 116, 15646–15659 CAS.
- J. Wang, Y. C. Song, Z. S. Li, J. D. Zhou, X. Y. Jing, M. L. Zhang and Z. H. Jiang, Energy Fuels, 2010, 24, 6463–6467 CrossRef CAS.
- Z. Wang, X. Zhang, J. H. Wang, L. D. Zou, Z. T. Liu and Z. P. Hao, J. Colloid Interface Sci., 2013, 396, 251–257 CrossRef CAS PubMed.
- Y. C. Song, J. Wang, Z. S. Li, D. H. Guan, T. Mann, Q. Liu and M. L. Zhang, Microporous Mesoporous Mater., 2012, 148, 159–165 CrossRef CAS PubMed.
- F. He, Z. B. Hu, K. Y. Liu, S. R. Zhang, H. T. Liu and S. B. Sang, J. Power Sources, 2014, 267, 188–196 CrossRef CAS PubMed.
- M. F. Shao, F. Y. Ning, Y. F. Zhao, J. W. Zhao, M. Wei, D. G. Evans and X. Duan, Chem. Mater., 2012, 24, 1192–1197 CrossRef CAS.
- J. B. Wei, J. Wang, Y. C. Song, Z. S. Li, Z. Gao, T. Mann and M. L. Zhang, J. Solid State Chem., 2012, 196, 175–181 CrossRef CAS PubMed.
- L. Yan, R. Y. Li, Z. J. Li, J. K. Liu, Y. J. Fang, G. L. Wang and Z. G. Gu, Electrochim. Acta, 2013, 95, 146–154 CrossRef CAS PubMed.
- B. Wang, Q. Liu, Z. Y. Qian, X. F. Zhang, J. Wang, Z. S. Li, H. J. Yan, Z. Gao, F. B. Zhao and L. H. Liu, J. Power Sources, 2014, 246, 747–753 CrossRef CAS PubMed.
- L. Yan, H. Kong and Z. J. Li, Acta Chim. Sin., 2013, 71, 822–828 CrossRef CAS.
- A. B. Béléké and M. Mizuhata, J. Power Sources, 2010, 195, 7669–7676 CrossRef PubMed.
- A. B. Béléké, E. Higuchi, H. Ionue and M. Mizuhata, J. Power Sources, 2013, 225, 215–220 CrossRef PubMed.
- J. Memon, J. H. Sun, D. L. Meng, W. Z. Ouyang, M. A. Memon, Y. Huang, S. K. Yan and J. X. Geng, J. Mater. Chem. A, 2014, 2, 5060–5067 CAS.
- Y. L. Niu, R. Y. Li, Y. J. Fang and J. K. Liu, Electrochim. Acta, 2013, 94, 360–366 CrossRef CAS PubMed.
- M. Li, J. P. Cheng, J. H. Fang, Y. Yang, F. Liu and X. B. Zhang, Electrochim. Acta, 2014, 134, 309–318 CrossRef CAS PubMed.
- Z. Gao, J. Wang, Z. S. Li, W. L. Yang, B. Wang, M. J. Hou, Y. He, Q. Liu, T. Mann, P. P. Yang, M. L. Zhang and L. H. Liu, Chem. Mater., 2011, 23, 3509–3516 CrossRef CAS.
- L. J. Zhang, J. Wang, J. J. Zhu, X. G. Zhang, K. S. Hui and K. N. Hui, J. Mater. Chem. A, 2013, 1, 9046–9053 CAS.
- J. Xu, S. L. Gai, N. Niu, P. Gao, Y. J. Chen and P. P. Yang, J. Mater. Chem. A, 2014, 2, 1022–1031 CAS.
- L. Li, R. M. Li, S. L. Gai, F. He and P. P. Yang, J. Mater. Chem. A, 2014, 2, 8758–8765 CAS.
- Y. Wimalasiri, R. Fan, X. S. Zhao and L. D. Zou, Electrochim. Acta, 2014, 134, 127–135 CrossRef CAS PubMed.
- M. Hu, Z. Y. Yang, L. X. Lei and Y. M. Sun, J. Power Sources, 2011, 196, 1569–1577 CrossRef CAS PubMed.
- Z. P. Xu, L. Li, C. Y. Cheng, R. G. Ding and C. H. Zhou, Appl. Clay Sci., 2013, 74, 102–108 CrossRef CAS PubMed.
- S. Werner, V. W. H. Lau, S. Hug, V. Duppel, H. C. Schaumann and B. V. Lotsch, Langmuir, 2013, 29, 9199–9207 CrossRef CAS PubMed.
- J. W. Zhao, J. L. Chen, S. M. Xu, M. F. Shao, D. P. Yan, M. Wei, D. G. Evans and X. Duan, J. Mater. Chem. A, 2013, 1, 8836–8843 CAS.
- M. F. Shao, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2011, 47, 3171–3173 RSC.
- M. A. Woo, M. S. Song, T. W. Kim, I. Y. Kim, J. Y. Ju, Y. S. Lee, S. J. Kim, J. H. Choy and S. J. Hwang, J. Mater. Chem., 2011, 21, 4286–4292 RSC.
- Y. H. Gu, Z. Y. Lu, Z. Chang, J. F. Liu, X. D. Lei, Y. P. Li and X. M. Sun, J. Mater. Chem. A, 2013, 1, 10655–10661 CAS.
- H. W. Park, J. S. Chae, S. M. Park, K. B. Kim and K. C. Roh, Met. Mater. Int., 2013, 19, 887–894 CrossRef CAS PubMed.
- J. W. Jiang, X. G. Zhang, L. H. Su, L. J. Zhang and F. Zhang, Chin. J. Inorg. Chem., 2010, 26, 1623–1628 CAS.
- J. W. Zhao, J. L. Chen, S. X. Xu, M. F. Shao, Q. Zhang, F. Wei, J. Ma, M. Wei, D. G. Evans and X. Duan, Adv. Funct. Mater., 2014, 24, 2938–2946 CrossRef CAS PubMed.
- L. H. Su, C. L. Ma, T. Hou and W. J. Han, RSC Adv., 2013, 3, 19807–19811 RSC.
- J. Yang, C. Yu, X. M. Fan, Z. Ling, J. S. Qiu and Y. Gogotsi, J. Mater. Chem. A, 2013, 1, 1963–1968 CAS.
- V. Gupta, S. Gupta and N. Miura, J. Power Sources, 2009, 189, 1292–1295 CrossRef CAS PubMed.
- J. B. Wei, Z. Gao, Y. C. Song, W. L. Yang, J. Wang, Z. S. Li, T. Mann, M. L. Zhang and L. H. Liu, Mater. Chem. Phys., 2013, 139, 395–402 CrossRef CAS PubMed.
- Z. P. Liu, R. Z. Ma, M. Osada, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2005, 127, 13869–13874 CrossRef CAS PubMed.
- (a) X. Liu, R. Ma, Y. Bando and T. Sasaki, Angew. Chem., Int. Ed., 2010, 49, 8253–8256 CrossRef CAS PubMed; (b) L. Liu, J. P. Cheng, J. Zhang, F. Liu and X. B. Zhang, J. Alloys Compd., 2014, 615, 868–874 CrossRef CAS PubMed.
- Y. L. Chen, Z. A. Hu, Y. Q. Chang, H. W. Wang, G. R. Fu, X. Q. Jin and L. J. Xie, Chin. J. Chem., 2011, 29, 2257–2262 CrossRef CAS PubMed.
- Z. Gao, W. L. Yang, Y. X. Yan, J. Wang, J. Ma, X. M. Zhang, B. H. Xing and L. H. Liu, Eur. J. Inorg. Chem., 2013, 4832–4838 CrossRef CAS PubMed.
- L. B. Kong, J. W. Lang, M. Liu, Y. C. Luo and L. Kang, J. Power Sources, 2009, 194, 1194–1201 CrossRef CAS PubMed.
- T. Wu and C. Z. Yuan, Mater. Lett., 2012, 85, 161–163 CrossRef CAS PubMed.
- L. R. Hou, C. Z. Yuan, L. Yang, L. F. Shen, F. Zhang and X. G. Zhang, CrystEngComm, 2011, 13, 6130–6135 RSC.
- J. Jiang, J. P. Liu, R. M. Ding, J. H. Zhu, Y. Y. Li, A. Z. Hu, X. Li and X. T. Huang, ACS Appl. Mater. Interfaces, 2011, 1, 99–103 Search PubMed.
- Z. Chen, Y. Chen, C. Zuo, S. Zhou, A. G. Xiao and A. X. Pan, Bull. Mater. Sci., 2013, 36, 239–244 CrossRef CAS PubMed.
- W. L. Yang, Z. Gao, J. Ma, J. Wang, X. M. Zhang and L. H. Liu, Dalton Trans., 2013, 42, 15706–15715 RSC.
- L. B. Kong, M. C. Liu, J. W. Lang, M. Liu, Y. C. Luo and L. Kang, J. Solid State Electrochem., 2011, 15, 571–577 CrossRef CAS.
- V. Gupta, T. Kusahara, H. Toyama, S. Gupta and N. Miura, Electrochem. Commun., 2007, 9, 2315–2319 CrossRef CAS PubMed.
- J. Tang, D. Q. Liu, Y. X. Zheng, X. W. Li, X. H. Wang and D. Y. He, J. Mater. Chem. A, 2014, 2, 2585–2591 CAS.
- J. Zhang, X. C. Wang, J. Ma, S. Liu and X. B. Yi, Electrochim. Acta, 2013, 104, 110–116 CrossRef CAS PubMed.
- C. M. Zhao, X. Wang, S. M. Wang, H. X. Wang, Y. C. Yang and W. T. Zheng, Mater. Res. Bull., 2013, 48, 3189–3195 CrossRef CAS PubMed.
- T. Zhao, H. Jiang and J. Ma, J. Power Sources, 2011, 196, 860–864 CrossRef CAS PubMed.
- C. M. Zhao, X. Wang, S. M. Wang, Y. Y. Wang, Y. X. Zhao and W. T. Zheng, Int. J. Hydrogen Energy, 2012, 37, 11846–11852 CrossRef CAS PubMed.
- G. X. Pan, X. Xia, F. Cao, P. S. Tang and H. F. Chen, Electrochim. Acta, 2012, 63, 335–340 CrossRef CAS PubMed.
- X. Ge, C. D. Gu, X. L. Wang and J. P. Tu, J. Phys. Chem. C, 2014, 118, 911–923 CAS.
- J. M. Ko, D. Soundarajan, J. H. Park, S. D. Yang, S. W. Kim, K. M. Kim and K. H. Yu, Curr. Appl. Phys., 2012, 12, 341–345 CrossRef PubMed.
- Z. A. Hu, Y. L. Xie, Y. X. Wang, L. J. Xie, G. R. Fu, X. Q. Jin, Z. Y. Zhang, Y. Y. Yang and H. Y. Wu, J. Phys. Chem. C, 2009, 113, 12502–12508 CAS.
- L. Wang, Z. H. Dong, Z. G. Wang, F. X. Zhang and J. Jin, Adv. Funct. Mater., 2013, 23, 2758–2764 CrossRef CAS PubMed.
- C. Y. Yan, H. Jiang, T. Zhao, C. Z. Li, J. Ma and P. S. Lee, J. Mater. Chem., 2011, 21, 10482–10488 RSC.
- D. T. Dam, X. Wang and J. M. Lee, RSC Adv., 2012, 2, 10512–10518 RSC.
- D. T. Dam and J. M. Lee, Nano Energy, 2013, 2, 1186–1196 CrossRef PubMed.
- B. Schneiderová, J. Demel, J. Pleštil, H. Tarábková, J. Bohuslav and K. Lang, Dalton Trans., 2014, 43, 10484–10491 RSC.
- J. P. Cheng, L. Liu, J. Zhang, F. Liu and X. B. Zhang, J. Electroanal. Chem., 2014, 722–723, 23–31 CrossRef CAS PubMed.
- C. G. Liu, Y. S. Lee, Y. J. Kim, I. C. Song and J. H. Kim, Synth. Met., 2009, 159, 2009–2012 CrossRef CAS PubMed.
- X. Hu, J. L. Jiang, Z. H. Fan and Z. Y. Ling, J. Electrochem. Soc., 2013, 160, 1425–1429 CrossRef PubMed.
- H. W. Park, Y. T. Ju, S. M. Park and K. C. Roh, RSC Adv., 2014, 4, 567–571 RSC.
- M. S. Wu and K. C. Huang, Int. J. Hydrogen Energy, 2011, 36, 13407–13413 CrossRef CAS PubMed.
- G. R. Fu, Z. A. Hu, L. J. Xie, X. Q. Jin, Y. X. Wang, Z. Y. Zhang, Y. Y. Yang and H. Y. Wu, Int. J. Electrochem. Sci., 2009, 4, 1052–1062 CAS.
- M. Aghazadeh, M. Ghaemi, B. Sabour and S. Dalvand, J. Solid State Electrochem., 2014, 18, 1569–1584 CrossRef CAS.
- K. Wang and L. Zhang, Electrochemical, 2013, 81, 259–261 CrossRef CAS PubMed.
- Y. X. Wang, Z. A. Hu and H. Y. Wu, Mater. Chem. Phys., 2011, 126, 580–583 CrossRef CAS PubMed.
- J. Liang, B. T. Dong, S. J. Ding, C. P. Li, B. Q. Li, J. Li and G. Yang, J. Mater. Chem. A, 2014, 2, 11299–11304 CAS.
- H. M. Du, L. F. Jiao, K. Z. Cao, Y. J. Wang and H. T. Yuan, ACS Appl. Mater. Interfaces, 2013, 5, 6643–6648 CAS.
- Q. L. Xian and J. Li, J. Inorg. Mater., 2010, 25, 1268–1272 CrossRef CAS.
- S. T. Xing, Q. Wang, Z. C. Ma, Y. S. Wu and Y. Z. Gao, Mater. Lett., 2012, 78, 99–101 CrossRef CAS PubMed.
- H. Jiang, T. Zhao, C. Z. Li and J. Ma, J. Mater. Chem., 2011, 21, 3818–3823 RSC.
- D. Ghosh, S. Giri, M. Mandal and C. K. Das, RSC Adv., 2014, 4, 26094–26101 RSC.
- X. Q. Tian, C. M. Cheng, L. Qian, B. Z. Zheng, H. Y. Yuan, S. P. Xie, D. Xiao and M. M. F. Choi, J. Mater. Chem., 2012, 22, 8029–8035 RSC.
- Y. Ren and L. Gao, J. Am. Ceram. Soc., 2010, 93, 3560–3564 CrossRef CAS PubMed.
- B. P. Bastakoti, H. S. Huang, L. C. Chen, K. C. W. Wu and Y. Yamauchi, Chem. Commun., 2012, 48, 9150–9152 RSC.
- G. Q. Yang, Y. Q. Wu, B. L. Wang, W. Y. Guo, Z. Y. Ren, Z. H. Bu, C. C. Miao and H. L. Li, J. Solid State Electrochem., 2012, 16, 3761–3767 CrossRef CAS.
- J. C. Huang, T. Lei, X. P. Wei, X. W. Liu, T. Liu, D. X. Cao, J. L. Yin and G. L. Wang, J. Power Sources, 2013, 232, 370–375 CrossRef CAS PubMed.
- Z. You, K. Shen, Z. C. Wu, X. F. Wang and X. H. Kong, Appl. Surf. Sci., 2012, 258, 8117–8123 CrossRef CAS PubMed.
- B. K. Kim, V. Chabot and A. P. Yu, Electrochim. Acta, 2013, 109, 370–380 CrossRef CAS PubMed.
- H. Fang, S. C. Zhang, T. Jiang, R. X. Lin and Y. Lin, Electrochim. Acta, 2014, 125, 427–434 CrossRef CAS PubMed.
- H. L. Cheng, A. D. Su, S. H. Li, S. T. Nguyen, L. Lu, C. Y. H. Lim and H. M. Duong, Chem. Phys. Lett., 2014, 601, 168–173 CrossRef CAS PubMed.
- J. T. Zhang, S. Liu, G. L. Pan, G. R. Li and X. P. Gao, J. Mater. Chem. A, 2014, 2, 1524–1529 CAS.
- L. N. Wang, H. Y. Chen, F. Cai and M. H. Chen, Mater. Lett., 2014, 115, 168–171 CrossRef CAS PubMed.
- J. Z. Fan, H. Y. Mi, Y. L. Xu and B. Gao, Mater. Res. Bull., 2013, 48, 1342–1345 CrossRef CAS PubMed.
- N. A. Alhebshi, R. B. Rakhi and H. N. Alshareef, J. Mater. Chem. A, 2013, 1, 14897–14903 CAS.
- J. W. Xiao and S. Yang, ChemPlusChem, 2012, 77, 807–816 CrossRef CAS PubMed.
- L. Q. Wang, X. C. Li, T. M. Guo, X. B. Yan and B. K. Tay, Int. J. Hydrogen Energy, 2014, 39, 7876–7884 CrossRef CAS PubMed.
- X. A. Chen, X. H. Chen, F. Q. Zhang, Z. Yang and S. M. Huang, J. Power Sources, 2013, 243, 555–561 CrossRef CAS PubMed.
- J. W. Zhu, S. Chen, H. Zhou and X. Wang, Nano Res., 2012, 5, 11–19 CrossRef CAS.
- F. Q. Zhang, D. Zhu, X. A. Chen, X. Xu, Z. Yang, C. Zou, K. Q. Yang and S. M. Huang, Phys. Chem. Chem. Phys., 2014, 16, 4186–4192 RSC.
- Z. H. Pu, Q. Liu, A. H. Qusti, A. M. Asiri, A. O. Al-Youbi and X. P. Sun, Electrochim. Acta, 2013, 109, 252–255 CrossRef CAS PubMed.
- J. P. Liu, C. W. Cheng, W. W. Zhou, H. X. Li and H. J. Fan, Chem. Commun., 2011, 47, 3436–3438 RSC.
- Q. Qu, S. Yang and X. Feng, Adv. Mater., 2011, 23, 5574–5580 CrossRef CAS PubMed.
- L. Cheng, H. Q. Li and Y. Y. Xia, J. Solid State Electrochem., 2006, 10, 405–410 CrossRef CAS PubMed.
- W. H. Jin, G. T. Cao and J. Y. Sun, J. Power Sources, 2008, 175, 686–691 CrossRef CAS PubMed.
- R. Barik, B. K. Jena, A. Dash and M. Mohapatra, RSC Adv., 2014, 4, 18827–18834 RSC.
- K. F. Chen, Y. D. Noh, W. Y. Huang, J. F. Ma and S. Komarneni, Ceram. Int., 2014, 40, 2877–2884 CrossRef CAS PubMed.
- Z. C. Li, M. Y. Guan, Z. S. Lou and T. M. Shang, Micro Nano Lett., 2012, 7, 33–36 Search PubMed.
- V. S. Jamadade, V. J. Fulari and C. D. Lokhande, J. Alloys Compd., 2011, 509, 6257–6261 CrossRef CAS PubMed.
- K. K. Lee, R. W. Y. Ng, K. K. She, W. S. Chin and C. H. Sow, Mater. Lett., 2014, 118, 150–153 CrossRef CAS PubMed.
- H. Xu, Z. A. Hu, A. L. Lu, L. Li, Y. Y. Yang, Z. Y. Zhang and H. Y. Wu, Mater. Chem. Phys., 2013, 141, 310–317 CrossRef CAS PubMed.
- Q. L. Shou, J. P. Cheng, L. Zhang, B. J. Nelson and X. B. Zhang, J. Solid State Chem., 2012, 185, 191–197 CrossRef CAS PubMed.
- X. F. Xia, W. Lei, Q. L. Hao, W. J. Wang, Y. X. Sun and X. Wang, Electrochim. Acta, 2013, 113, 117–126 CrossRef CAS PubMed.
- S. Anandan, B. G. S. Raj, G. J. Lee and J. J. Wu, Mater. Res. Bull., 2013, 48, 3357–3361 CrossRef CAS PubMed.
- J. S. Liu, Y. Hu, T. L. Chuang and C. L. Huang, Thin Solid Films, 2013, 544, 186–190 CrossRef CAS PubMed.
- K. V. Gurav, U. M. Patil, S. W. Shin, G. L. Agawane, M. P. Suryawanshi, S. M. Pawar, P. S. Patil, C. D. Lokhande and J. H. Kim, J. Alloys Compd., 2013, 573, 27–31 CrossRef CAS PubMed.
- J. C. Huang, T. Liu, X. W. Liu, L. F. Du, D. X. Cao, J. L. Yin and G. L. Wang, J. Electroanal. Chem., 2013, 696, 15–19 CrossRef CAS PubMed.
- G. K. Wang, X. Sun, F. Y. Lu, Q. K. Yu, C. S. Liu and J. Lian, J. Solid State Chem., 2012, 185, 172–179 CrossRef CAS PubMed.
- (a) X. F. Gong, J. P. Cheng, F. Liu, L. Zhang and X. B. Zhang, J. Power Sources, 2014, 267, 610–616 CrossRef CAS PubMed; (b) B. B. Wang, X. X. Fu, F. Liu, S. L. Shi, J. P. Cheng and X. B. Zhang, J. Alloys Compd., 2014, 587, 82–89 CrossRef CAS PubMed.
- (a) H. Chen, S. X. Zhou and L. M. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 8621–8630 CrossRef CAS PubMed; (b) J. P. Cheng, B. B. Wang, M. G. Zhao, F. Liu and X. B. Zhang, Sens. Actuators, B, 2014, 190, 78–85 CrossRef CAS PubMed.
- L. Huang, D. C. Chen, Y. Ding, Z. L. Wang, Z. Z. Zeng and M. L. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 11159–11162 CAS.
- K. B. Xu, R. J. Zou, W. Y. Li, Q. Liu, X. J. Liu, L. An and J. Q. Hu, J. Mater. Chem. A, 2014, 2, 10090–10097 CAS.
- J. H. Zhong, A. L. Wang, G. R. Li, J. W. Wang, Y. N. Ou and Y. X. Tong, J. Mater. Chem., 2012, 22, 5656–5665 RSC.
- C. Guan, J. P. Liu, C. W. Cheng, H. X. Li, X. L. Li, W. W. Zhou, H. Zhang and H. J. Fan, Energy Environ. Sci., 2011, 4, 4496–4499 CAS.
- C. H. Tang, X. S. Yin and H. Gong, ACS Appl. Mater. Interfaces, 2013, 5, 10574–10582 CAS.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, J. Chen, X. L. Wang, C. D. Gu, C. Guan, J. S. Luo and H. J. Fan, Chem. Mater., 2012, 24, 3793–3799 CrossRef CAS.
- F. Y. Ning, M. F. Shao, C. L. Zhang, S. M. Xu, M. Wei and X. Duan, Nano Energy, 2014, 7, 134–142 CrossRef CAS PubMed.
- H. Yi, X. Chen, H. W. Wang and X. F. Wang, Electrochim. Acta, 2014, 116, 372–378 CrossRef CAS PubMed.
- A. Ramadoss and S. J. Kim, Electrochim. Acta, 2014, 136, 105–111 CrossRef CAS PubMed.
- W. J. Zhou, X. J. Liu, Y. H. Sang, Z. H. Zhao, K. Zhou, H. Liu and S. W. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 4578–4586 CAS.
- J. Q. Sun, W. Y. Li, B. J. Zhang, G. Li, L. Jiang, Z. G. Chen, R. J. Zou and J. Q. Hu, Nano Energy, 2014, 4, 56–64 CrossRef CAS PubMed.
- J. L. Kang, A. Hirata, H. J. Qiu, L. Y. Chen, X. B. Ge, T. Fujita and M. W. Chen, Adv. Mater., 2014, 26, 269–272 CrossRef CAS PubMed.
- G. Yu, X. Xie, L. Pan, Z. Bao and Y. Cui, Nano Energy, 2013, 2, 213–234 CrossRef CAS PubMed.
- N. W. Duffy, W. Baldsing and A. G. Pandolfo, Electrochim. Acta, 2008, 54, 535–539 CrossRef CAS PubMed.
- F. Wang, S. Xiao, Y. Hou, C. Hu, L. Liu and Y. Wu, RSC Adv., 2013, 3, 13059–13084 RSC.
- A. B. Yuan and Q. L. Zhang, Electrochem. Commun., 2006, 8, 1173–1178 CrossRef CAS PubMed.
- Y. Y. Liang, H. L. Li and X. G. Zhang, Mater. Sci. Eng., A, 2008, 473, 317–322 CrossRef PubMed.
- H. B. Kong, M. Liu, J. W. Lang, Y. C. Luo and L. Kang, J. Electrochem. Soc., 2009, 156, 1000–1004 CrossRef PubMed.
- F. S. Fedorov, J. Linnemann, K. Tschulik, L. Giebeler, M. Uhlemann and A. Gebert, Electrochim. Acta, 2013, 90, 166–170 CrossRef CAS PubMed.
- C. H. Chen, D. S. Tsai, W. H. Chung, K. Y. Lee, Y. M. Chen and Y. S. Huang, J. Power Sources, 2012, 205, 510–515 CrossRef CAS PubMed.
- C. Qian, T. Jie, S. Y. Norio and Q. L. Chang, Sci. Technol. Adv. Mater., 2014, 15, 14206–14211 CrossRef.
- Y. Tian, J. W. Yan, L. P. Huang, R. Xue, L. X. Hao and B. L. Yi, Mater. Chem. Phys., 2014, 143, 1164–1170 CrossRef CAS PubMed.
- J. C. Huang, P. P. Xu, D. X. Cao, X. B. Zhou, S. N. Yang, Y. J. Li and G. L. Wang, J. Power Sources, 2014, 246, 371–376 CrossRef CAS PubMed.
- J. Y. Ji, L. L. Zhang, H. X. Ji, Y. Li, X. Zhao, X. Bai, X. B. Fan, F. B. Zhang and R. S. Ruoff, ACS Nano, 2013, 7, 6237–6243 CrossRef CAS PubMed.
- Z. Tang, C. H. Tang and H. Gong, Adv. Funct. Mater., 2012, 22, 1272–1278 CrossRef CAS PubMed.
- X. Wang, J. Y. Liu, Y. Y. Wang, C. M. Zhao and W. T. Zheng, Mater. Res. Bull., 2014, 52, 89–95 CrossRef CAS PubMed.
- Y. G. Wang, D. D. Zhou, D. Zhao, M. Y. Hou, C. X. Wang and Y. Y. Xia, J. Electrochem. Soc., 2013, 160, 98–104 CrossRef PubMed.
- J. Yan, Z. J. Fan, W. Sun, G. Q. Ning, T. Wei, Q. Zhang, R. F. Zhang, L. J. Zhi and F. Wei, Adv. Funct. Mater., 2012, 22, 2632–2641 CrossRef CAS PubMed.
- H. L. Wang, Y. Y. Liang, T. Mirfakhrai, Z. Chen, H. S. Casalongue and H. J. Dai, Nano Res., 2011, 4, 729–736 CrossRef CAS PubMed.
- J. W. Lang, L. B. Kong, M. Liu, Y. C. Luo and L. Kang, J. Solid State Electrochem., 2010, 14, 1533–1539 CrossRef CAS.
- J. H. Park, S. W. Kim, O. O. Park and J. M. Ko, Appl. Phys. A, 2006, 82, 593–597 CrossRef CAS PubMed.
- X. X. Liu, C. Wang, Y. B. Dou, A. Zhou, T. Pan, J. B. Han and M. Wei, J. Mater. Chem. A, 2014, 2, 1682–1685 CAS.
- W. F. Zhang, C. Ma, J. H. Fang, J. P. Cheng, X. B. Zhang, S. R. Dong and L. Zhang, RSC Adv., 2013, 3, 2483–2490 RSC.
- X. Wang, A. Sumboja, M. F. Lin, J. Yan and P. S. Lee, Nanoscale, 2012, 4, 7266–7272 RSC.
- H. L. Wang, Q. M. Gao and J. Hu, J. Power Sources, 2010, 195, 3017–3024 CrossRef CAS PubMed.
- C. C. Hu, J. C. Chen and K. H. Chang, J. Power Sources, 2013, 221, 128–133 CrossRef CAS PubMed.
- C. H. Lien, C. C. Hu, C. T. Hsu and D. S. H. Wong, Electrochem. Commun., 2013, 34, 323–326 CrossRef CAS PubMed.
- C. C. Hu, C. T. Hsu, K. H. Chang and H. Y. Hsu, J. Power Sources, 2013, 238, 180–189 CrossRef CAS PubMed.
- Z. H. Sun and A. B. Yuan, Chin. J. Chem. Eng., 2009, 17, 150–155 CrossRef CAS.
- A. Shanmugavani and R. K. Selvan, RSC Adv., 2014, 4, 27022–27029 RSC.
- I. Shakir, Z. Ali, J. Bae, J. Park and D. J. Kang, RSC Adv., 2014, 4, 6324–6329 RSC.
| This journal is © The Royal Society of Chemistry 2014 |



