Sandwich-like graphene nanosheets decorated with superparamagnetic CoFe2O4 nanocrystals and their application as an enhanced electromagnetic wave absorber
Abstract
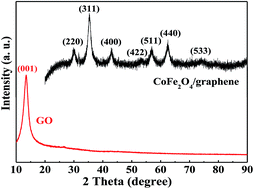
Sandwich-structured CoFe2O4/graphene hybrids are fabricated through a facile one-pot polyol route, and the electromagnetic wave absorption properties are investigated. TEM images and elemental mapping indicate that the graphene nanosheets are decorated with numerous tiny CoFe2O4 nanocrystals with a relatively uniform size of 7.8 nm, forming sandwich-like nanostructures. Magnetization measurement reveals that the CoFe2O4/graphene hybrids possess superparamagnetism at room temperature with zero coercivity. Investigations of the electromagnetic properties indicate that the complex permittivity of CoFe2O4/graphene hybrids is significantly improved in comparison with that of pure CoFe2O4 nanocrystals, leading to enhanced electromagnetic wave absorption properties of the CoFe2O4/graphene hybrids. The maximum reflection loss of the CoFe2O4/graphene hybrids is up to −36.4 dB at 12.9 GHz with the matching thickness of 2.5 mm, and the absorption bandwidth with reflection loss values below −10 dB is in the range of 5.4–18 GHz when the matching thicknesses are only 1.5–4.0 mm. These results suggest that the sandwich-like CoFe2O4/graphene hybrids with enhanced electromagnetic wave absorption properties and wide absorption bandwidth are an ideal candidate for electromagnetic wave absorption applications in the future.

 Please wait while we load your content...
Please wait while we load your content...