DOI:
10.1039/C4RA06673A
(Paper)
RSC Adv., 2014,
4, 44089-44099
Binder effect on the battery performance of mesoporous copper ferrite nanoparticles with grain boundaries as anode materials†
Received
4th July 2014
, Accepted 19th August 2014
First published on 19th August 2014
Abstract
Mesoporous copper ferrite (CuFe2O4) nanoparticles were prepared by citric acid and urea assisted combustion process. The local chemical modification obtained through addition of acid and base to the synthesis process, modifies the combustion reaction and it affects the crystalline phase, pore size and surface area of CuFe2O4 nanoparticles. The effect of grain boundary surfaces with different pore sizes on the electrical properties was explained using impedance spectroscopy results, and it was also reflected in the battery performance. The CR 2032 coin cells were fabricated using mesoporous CuFe2O4 nanoparticles with polyvinylidene difluoride (PVdF)/sodium alginate as binders; moreover, the effect of PVdF and alginate binders on the battery performance and coulombic efficiency were analyzed and were correlated to the area covered under oxidation and reduction cycles of cyclic voltammetry. CR 2032 coin cells were fabricated using mesoporous CuFe2O4 raw, acid and base samples with alginate binder and cycled at 1C rate to obtain the effect of crystallite size and surface area on the battery performance. The addition of alginate binder and its good adhesive nature improves the battery performance and retains the capacity for longer cycles.
1. Introduction
Porous materials find applications in different emerging fields like catalysts, selective gas adsorption and purification systems, drug delivery, electrode materials for lithium ion batteries, and fuel cells. Most of the applications solely depend on the pore size and total surface area of the porous materials.1–5 In particular, porous anode materials can enhance capacity, which is even greater than the theoretical capacity of the anode material and improves the charge/discharge rate capability of lithium ion batteries.6 Metal oxides like ferrites were introduced as alternate anode materials to meet the high energy demands and also to overcome the short circuit problem existing in lithium anode.7–10 Nanostructured spinel ferrites have been developed and studied as anode materials for lithium ion batteries because of their improved Li-ion-storage mechanism. The charge and discharge reaction mechanism differs from the classical Li ion insertion/extraction and Li alloying/dealloying processes. It involves the formation and decomposition of lithium oxide (Li2O), along with the reduction and oxidation of metal nanoparticles.11,12 The charge and discharge reaction rates depends on the availability of electrochemical reaction sites, which is more in nanomaterials as well as in porous materials, where the number of atoms on the exposed surface is more. Apart from the positive aspects of large reaction sites, the possible side reactions between electrolyte and electrode surfaces and better electrical contact between electrode materials and current collectors also influence the battery performance.13,14 Recent literature reports also iterate the importance of different binders' role on the battery performance.15–20 Hence, the application of porous anode materials with good binders will be the appropriate choice to improve the lithium ion battery performance. Porous materials with different pore size were prepared by using surfactant, soft template and hard template mediated synthesis routes; however, the complete removal of additives from the parent material is quite difficult in the templated synthesis routes. In our present study, mesoporous copper ferrite nanoparticles were prepared through citric acid and urea assisted combustion process and the surface area and pore size were modified through simple chemical modification by adding small quantities of acid or base to the citric acid and urea assisted combustion process. The CR 2032 coin cells were fabricated using mesoporous CuFe2O4 with polyvinylidene difluoride (PVdF) and sodium alginate as binders and the battery performance and electrochemical reactions were studied using a battery life cycle tester and cyclic voltammogram. The battery performance including coulombic efficiency of the battery fabricated with alginate binders was compared with the battery fabricated with PVdF binders. The alginate binder plays an important role in maintaining better electrical contact between electrode, current collector and carbon black and also minimizes the side reaction between electrolyte and electrodes and maintains the capacity for longer cycles.
2. Experimental
Mesoporous CuFe2O4 nanoparticles were prepared by using citric acid and urea assisted combustion process by maintaining the metal ions![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid
citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea molar ratio as (M
urea molar ratio as (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U) = 1
U) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. The local chemical conditions of the combustion process were modified by adding excess acid or base. AR grade cupric nitrate trihydrate, ferric nitrate nanohydrate from Sigma Aldrich, India (99.9% purity), citric acid and urea from Fisher Scientific, India (99.9% purity) were used as precursor materials for the synthesis. The required cupric nitrate trihydrate (5 g) and ferric nitrate nonahydrate (16.9 g) were dissolved each in 100 ml distilled water and sonicated for 10 minutes in an ultrasonic probe type sonicator with a frequency of 40 kHz to obtain complete and homogeneous dissolution. Later, the mixture of cupric nitrate and ferric nitrate solutions were sonicated for 10 minutes and stirred at 353 K. The required citric acid (12 g) and urea (1.8 g) were mixed with 100 ml distilled water and added to the above solution under continuous stirring after 30 minutes, and the sample was labeled as CuFe2O4 raw. In another two sets of samples, 2 ml of concentrated nitric acid or 2 ml of ammonia solution was added in excess to the CuFe2O4 raw solution with continuous stirring and labeled as CuFe2O4 acid and CuFe2O4 base, respectively. CuFe2O4 raw, CuFe2O4 acid and CuFe2O4 base represent the sample preparation conditions of the combustion process and were maintained throughout the manuscript. Water evaporates from the solution mixture with continuous stirring at 353 K and polymeric resins were formed. The polymeric resins were dried in an oven at 333 K for 24 h and slow release of CO2 and ammonia gas vapors from the polymeric resin forms dried porous polymeric resins with volume expansion called polymeric intermediates. The addition of small quantities of acid and base changed the local chemical conditions and also changed the crystallinity of the byproduct formed in the polymeric intermediate. The volume expansion of the CuFe2O4 base PI was small compared to CuFe2O4 raw and acid PI. The polymeric intermediates were calcined at 443 and 623 K for 3 h under atmospheric conditions and mesoporous CuFe2O4 nanoparticles were obtained. The complete process was monitored using powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), scanning electron microscopy and energy dispersive X-ray spectrometry (SEM-EDS). The XRD spectra were obtained using a PANalytical XpertPRO diffractometer with Cu Kα radiation of wavelength 0.15418 nm from 10°–80° at a scan rate of 1.5° per minute. Fourier transform infrared spectra were recorded on a Shimadzu FTIR/8300/8700 spectrophotometer in the frequency range of 4000–400 cm−1 with a 2 cm−1 resolution for 20 scans. FTIR spectra were recorded using thin transparent pellets and each pellet was made by pressing an approximate mixture of 0.5 mg sample and 10 mg of spectra-pure KBr. The thermal behaviors of the prepared samples were analyzed using a TA Q20DSC differential scanning calorimeter under nitrogen gas flow. The particle size and morphology and elemental mapping of CuFe2O4 samples were analyzed using a Hitachi SN 3400N SEM and JEOL 6701 FESEM instruments. The FETEM images and selected area diffraction (SAED) pattern were taken using JEOL, JEM 2100F FETEM instrument. The pore size, surface area and pore size distribution were analyzed using Micromeritics GEMINI VII BET surface area analyzer. For impedance analysis, the samples were ground into fine powder and the samples were made into pellets of dimension 10 mm diameter and 1.5–2 mm thickness using a spectra Lab pelletizer by applying 5 ton pressure. The pellets were coated with silver paste for better electrical contact and maintained between silver electrodes for impedance measurement. The impedance measurements were made by applying an AC signal of 1 V with a frequency range from 1 Hz to 10 MHz using a Novacontrol Alpha AT high performance frequency response analyzer. The lithium ion battery electrodes were prepared by using active electrode material, carbon black, sodium alginate or PVdF as binders. The active material, carbon black and binders weight ratio was maintained at 70
0.5. The local chemical conditions of the combustion process were modified by adding excess acid or base. AR grade cupric nitrate trihydrate, ferric nitrate nanohydrate from Sigma Aldrich, India (99.9% purity), citric acid and urea from Fisher Scientific, India (99.9% purity) were used as precursor materials for the synthesis. The required cupric nitrate trihydrate (5 g) and ferric nitrate nonahydrate (16.9 g) were dissolved each in 100 ml distilled water and sonicated for 10 minutes in an ultrasonic probe type sonicator with a frequency of 40 kHz to obtain complete and homogeneous dissolution. Later, the mixture of cupric nitrate and ferric nitrate solutions were sonicated for 10 minutes and stirred at 353 K. The required citric acid (12 g) and urea (1.8 g) were mixed with 100 ml distilled water and added to the above solution under continuous stirring after 30 minutes, and the sample was labeled as CuFe2O4 raw. In another two sets of samples, 2 ml of concentrated nitric acid or 2 ml of ammonia solution was added in excess to the CuFe2O4 raw solution with continuous stirring and labeled as CuFe2O4 acid and CuFe2O4 base, respectively. CuFe2O4 raw, CuFe2O4 acid and CuFe2O4 base represent the sample preparation conditions of the combustion process and were maintained throughout the manuscript. Water evaporates from the solution mixture with continuous stirring at 353 K and polymeric resins were formed. The polymeric resins were dried in an oven at 333 K for 24 h and slow release of CO2 and ammonia gas vapors from the polymeric resin forms dried porous polymeric resins with volume expansion called polymeric intermediates. The addition of small quantities of acid and base changed the local chemical conditions and also changed the crystallinity of the byproduct formed in the polymeric intermediate. The volume expansion of the CuFe2O4 base PI was small compared to CuFe2O4 raw and acid PI. The polymeric intermediates were calcined at 443 and 623 K for 3 h under atmospheric conditions and mesoporous CuFe2O4 nanoparticles were obtained. The complete process was monitored using powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), scanning electron microscopy and energy dispersive X-ray spectrometry (SEM-EDS). The XRD spectra were obtained using a PANalytical XpertPRO diffractometer with Cu Kα radiation of wavelength 0.15418 nm from 10°–80° at a scan rate of 1.5° per minute. Fourier transform infrared spectra were recorded on a Shimadzu FTIR/8300/8700 spectrophotometer in the frequency range of 4000–400 cm−1 with a 2 cm−1 resolution for 20 scans. FTIR spectra were recorded using thin transparent pellets and each pellet was made by pressing an approximate mixture of 0.5 mg sample and 10 mg of spectra-pure KBr. The thermal behaviors of the prepared samples were analyzed using a TA Q20DSC differential scanning calorimeter under nitrogen gas flow. The particle size and morphology and elemental mapping of CuFe2O4 samples were analyzed using a Hitachi SN 3400N SEM and JEOL 6701 FESEM instruments. The FETEM images and selected area diffraction (SAED) pattern were taken using JEOL, JEM 2100F FETEM instrument. The pore size, surface area and pore size distribution were analyzed using Micromeritics GEMINI VII BET surface area analyzer. For impedance analysis, the samples were ground into fine powder and the samples were made into pellets of dimension 10 mm diameter and 1.5–2 mm thickness using a spectra Lab pelletizer by applying 5 ton pressure. The pellets were coated with silver paste for better electrical contact and maintained between silver electrodes for impedance measurement. The impedance measurements were made by applying an AC signal of 1 V with a frequency range from 1 Hz to 10 MHz using a Novacontrol Alpha AT high performance frequency response analyzer. The lithium ion battery electrodes were prepared by using active electrode material, carbon black, sodium alginate or PVdF as binders. The active material, carbon black and binders weight ratio was maintained at 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 for making electrode material slurries. Doubly distilled water was used as a solvent for making slurry using alginate binder, whereas N-methyl-2-pyrrolidone (NMP) solution was used for making slurry using PVdF binder. The coated slurries on a copper foil were dried and used as working electrodes, lithium foil was used as a reference electrode and Celgard polymer membrane was used as separator. 1 M LiPF6 dissolved in EC/DMC (1
10 for making electrode material slurries. Doubly distilled water was used as a solvent for making slurry using alginate binder, whereas N-methyl-2-pyrrolidone (NMP) solution was used for making slurry using PVdF binder. The coated slurries on a copper foil were dried and used as working electrodes, lithium foil was used as a reference electrode and Celgard polymer membrane was used as separator. 1 M LiPF6 dissolved in EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ratio was used as electrolyte. The CR 2032 coin cells were assembled in an argon filled VAC glove box using the prepared anode material. The cyclic voltammogram measurement was made for the prepared CR 2032 coin cells using CH instruments between 3 and 0.002 V at a scan rate of 0.2 mV s−1. The charge and discharge measurements were performed using a Bitrode battery life cycle tester between 3 V and 0.002 V at 0.1 C and 1 C rates.
1) ratio was used as electrolyte. The CR 2032 coin cells were assembled in an argon filled VAC glove box using the prepared anode material. The cyclic voltammogram measurement was made for the prepared CR 2032 coin cells using CH instruments between 3 and 0.002 V at a scan rate of 0.2 mV s−1. The charge and discharge measurements were performed using a Bitrode battery life cycle tester between 3 V and 0.002 V at 0.1 C and 1 C rates.
3. Result and discussion
3.1. X-ray diffraction
Fig. 1a shows the powder X-ray diffraction patterns for the CuFe2O4 raw, acid and base polymeric intermediates (PI) dried at 333 K. The observed X-ray diffraction patterns for the CuFe2O4 raw PI shows the formation of a crystalline tetragonal ammonium nitrate phase, which is confirmed by comparing the observed diffraction pattern with JCPDS data. The observed peak free diffraction pattern of CuFe2O4 acid PI shows the formation of an amorphous phase. The observed diffraction pattern of CuFe2O4 base PI shows the formation of orthorhombic ammonium nitrate, which is confirmed by comparing the observed diffraction pattern with JCPDS data. In the synthesis process, citric acid and urea were added as fuels. When urea is added to the citric acid and metal nitrate solution mixture, it reacts with the H+ ion and releases CO2 gas and forms NH4+ ion. NH4+ ion reacts with nitrates from the precursors and forms ammonium nitrate.21 The addition of excess acid/base modified the local chemical conditions and inhibits/favors the formation of crystalline ammonium nitrate. The formation of ammonium nitrate was confirmed from the XRD results. Fig. 1b shows the powder X-ray diffraction patterns of CuFe2O4 raw, acid and base polymeric intermediates (PI) calcined at 623 K. The powder X-ray diffraction patterns of CuFe2O4 raw and acid polymeric intermediates calcined at 623 K showed the diffraction patterns, corresponding to the formation of cubic CuFe2O4 crystalline phases, which are confirmed by comparing the observed diffraction pattern with JCPDS data. In general, the formation of a cubic CuFe2O4 phase was observed at low temperature and the formation of a tetragonal CuFe2O4 phase was observed at high temperature. In our synthesis, mixed tetragonal and cubic phase was observed in CuFe2O4 base sample calcined at 623 K which was due to large heat generation through decomposition of orthorhombic ammonium nitrate during combustion. The same result was also reflected in the DSC curve. Due to poor volume expansion of CuFe2O4 base polymeric intermediate, the released heat was completely felt by the sample, which favored the formation of mixed cubic and tetragonal CuFe2O4 phase with increased crystallite size. A CuFe2O4 crystalline phase was formed at 448 K; however, in order to remove the undecomposed organic derivatives observed in FTIR spectrum, the sample was further calcined at 623 K. From the observed diffraction peaks, the average crystallite size and strain values were calculated for CuFe2O4 raw, acid and base samples using Williamson-Hall (WH) plots, which are shown in Fig. 2a–c. The average crystallite sizes are 8.59, 16.92 and 91.2 nm, respectively, for CuFe2O4 raw, acid and base samples obtained at 623 K.
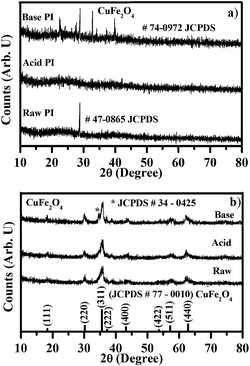 |
| | Fig. 1 Powder X-ray diffraction patterns of CuFe2O4 raw, acid and base PI samples (a) dried at 333 K (b) calcined at 623 K. | |
 |
| | Fig. 2 Williamson-Hall plots for CuFe2O4 (a) raw (b) acid (c) base samples obtained at 623 K. | |
3.2. Fourier transform infrared spectroscopy
Fig. 3a shows the FTIR spectra of nanocrystalline CuFe2O4 raw, acid and base PI samples dried at 333 K. From Fig. 3a, the FTIR spectra of CuFe2O4 raw PI sample obtained at 333 K showed the vibration bands at 3430, 3173, 1725, 1616, 1550, 1440, 1384 and 1315 cm−1. The observed characteristic vibration bands at 3430 and 1616 cm−1 correspond to the stretching and bending modes of water molecules. The band at 3173 cm−1 corresponds to NH3 vibration from ammonia groups. The bands at 1550 and 1440 cm−1 correspond to the symmetric and asymmetric stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, which confirm the chelation of metal ions by citric acid. The band at 1725 cm−1 corresponds to the asymmetric vibrations of bridging COO− groups and the corresponding symmetric vibration observed at 1315 cm−1. Similar vibration bands are observed for CuFe2O4 acid and base PI samples. A band at 1384 cm−1 shows the presence of NO3− groups.22,23 The observed IR bands confirm the citric acid polymerization, chelation of metal ions and presence of nitrate and ammonia in the PI. The addition of excess acid and base in the reaction was reflected in the corresponding high intense vibration bands of nitrates and ammonia in the CuFe2O4 acid and base PI samples observed at 3173 and 1384 cm−1, respectively. Fig. 3b shows the FTIR spectra of CuFe2O4 raw, acid and base PI samples calcined at 623 K. During calcination, the organic impurities were decomposed from the sample, which is reflected in the absence of corresponding IR bands shown in Fig. 3b. The observed vibration bands at 590 and 414 cm−1 correspond to the octahedral and tetrahedral vibration of ferrites.24 By comparing the observed vibration bands of all CuFe2O4 samples calcined at 623 K, CuFe2O4 raw sample shows organic impurity free vibration bands corresponding to CuFe2O4. Hence, the FTIR results confirmed the purity of CuFe2O4 sample prepared using citric acid urea assisted combustion process.
O bonds, which confirm the chelation of metal ions by citric acid. The band at 1725 cm−1 corresponds to the asymmetric vibrations of bridging COO− groups and the corresponding symmetric vibration observed at 1315 cm−1. Similar vibration bands are observed for CuFe2O4 acid and base PI samples. A band at 1384 cm−1 shows the presence of NO3− groups.22,23 The observed IR bands confirm the citric acid polymerization, chelation of metal ions and presence of nitrate and ammonia in the PI. The addition of excess acid and base in the reaction was reflected in the corresponding high intense vibration bands of nitrates and ammonia in the CuFe2O4 acid and base PI samples observed at 3173 and 1384 cm−1, respectively. Fig. 3b shows the FTIR spectra of CuFe2O4 raw, acid and base PI samples calcined at 623 K. During calcination, the organic impurities were decomposed from the sample, which is reflected in the absence of corresponding IR bands shown in Fig. 3b. The observed vibration bands at 590 and 414 cm−1 correspond to the octahedral and tetrahedral vibration of ferrites.24 By comparing the observed vibration bands of all CuFe2O4 samples calcined at 623 K, CuFe2O4 raw sample shows organic impurity free vibration bands corresponding to CuFe2O4. Hence, the FTIR results confirmed the purity of CuFe2O4 sample prepared using citric acid urea assisted combustion process.
 |
| | Fig. 3 FTIR spectra of CuFe2O4 raw, acid and base PI samples (a) dried at 333 K (b) calcined at 623 K. | |
3.3. Differential scanning calorimetry
Fig. 4 shows the DSC curves for CuFe2O4 raw, acid and base polymeric intermediate samples recorded between 300 K and 773 K with an inset figure of the expanded portion of DSC curves between 400 K and 500 K. The DSC thermogram shows one exothermic peak that corresponds to the decomposition reaction during combustion. From Fig. 4, CuFe2O4 raw, acid and base PI samples show ignition temperatures at 460, 462 and 476 K, respectively. The change in ignition temperature as well as the broadness of the exothermic peak reflects the combustion reaction, which is modified based on the addition of excess acid or base to the citric acid and urea assisted combustion process. The CuFe2O4 raw PI sample shows a small exothermic peak, which corresponds to the decomposition of citric acid, whose decomposition temperature is around 460 K. The addition of excess acid increases the oxidant and modifies the fuel to oxidant ratio, which is reflected in the heat evolution of the exothermic peak. The exothermic peak observed in CuFe2O4 base PI sample at 476 K corresponds to the decomposition of ammonium nitrate, whose decomposition temperature is around 475 K. The heat evolved during the combustion reaction determines the crystallite size and corresponding surface area of the samples and it is concurrent with the XRD and BET results.
 |
| | Fig. 4 DSC curves for CuFe2O4 raw, acid and base polymeric intermediates (PI) (inset: expanded portion of DSC curve between 400 and 500 K). | |
3.4. Field emission scanning electron microscopy and scanning electron microscopy-energy dispersive X-ray spectroscopy
Fig. 5a–c show the SEM images of CuFe2O4 raw, acid and base PI samples dried at 333 K, respectively. Fig. 6a–d show the FESEM images of CuFe2O4 raw, acid and base samples obtained at 623 K and SEM-EDS spectrum of CuFe2O4 raw sample obtained at 623 K, respectively. The citric acid and urea assisted combustion process released CO2, NH3 and H2O vapors during polymerization. The released gas acts as gas templates and produces a lot of pores in the dried polymeric intermediate sample, which were shown in Fig. 5a–c.25,26 During combustion reaction, the removal of gaseous products from the PI retains the cavity in the parent material. The CuFe2O4 particles shown in Fig. 6a–c have spherical morphology with agglomeration and the particle size was varied from 30 to 150 nm. The elemental spectrum results confirmed the presence and uniform distribution of Cu, Fe and O elements in the CuFe2O4 raw sample. Hence, SEM, FESEM, SEM-EDS results of CuFe2O4 sample obtained at 623 K confirmed the formation of CuFe2O4 nanoparticles.
 |
| | Fig. 5 SEM images of CuFe2O4 (a) raw (b) acid (c) base polymeric intermediates (PI) dried at 333 K. | |
 |
| | Fig. 6 FESEM images of CuFe2O4 (a) raw (b) acid (c) base (d) SEM-EDS spectrum of CuFe2O4 Raw samples obtained at 623 K. | |
3.5. Field emission transmission electron microscopy and selected area diffraction pattern (FETEM-SAED)
Fig. 7 shows the FETEM images of CuFe2O4 raw sample obtained at 623 K taken at different magnifications and the corresponding selected area electron diffraction pattern. The FETEM images clearly showed the formation of nanoparticles oriented in different directions with sizes less than 100 nm. The high-resolution FETEM image shows the lattice fringe pattern with an inter-planar spacing of 2.5 Å corresponding to (311) plane of crystalline cubic CuFe2O4. The formation of a crystalline pattern is confirmed from the selected area electron diffraction pattern, which agrees with the cubic crystalline CuFe2O4. Hence, FETEM and SAED pattern confirms the formation of CuFe2O4 nanoparticles with grain boundaries.
 |
| | Fig. 7 FETEM images of CuFe2O4 raw sample obtained at 623 K taken at different magnifications and SAED pattern for CuFe2O4 raw sample obtained at 623 K. | |
3.6. N2 adsorption/desorption isotherm
Fig. 8a–c, 9a–c and 10a–c show the N2 adsorption/desorption isotherm curves, pore diameter distribution and 1/[Q(P/Po − 1)] vs. relative pressure (P/Po) plots for the CuFe2O4 raw, acid and base samples obtained at 623 K, respectively. The entire isotherm patterns were compared with IUPAC standard isotherm pattern and the observed isotherms correspond to mesoporous type.27 The average crystallite size, strain values calculated from Williamson-Hall (WH) plots and pore size, total surface area, pore volume, surface area due to pores evaluated from BET method are listed in Table 1. By comparing the results tabulated in Table 1, the lowest crystallite size and high surface area was obtained for CuFe2O4 raw sample. This indicates that it has high grain boundary surfaces. The crystallite size variation is attributed to the heat evolved during the combustion reaction and the same results were confirmed through XRD and DSC results. The measured pore size is in the increasing order for the CuFe2O4 raw, acid and base samples. The origin of pores in the CuFe2O4 samples is due to the cavity formation from the removal of additives and byproducts during combustion process. Low crystallite size, high surface area and high surface energy were obtained for CuFe2O4 raw sample, which was considered for battery fabrication and further electrochemical studies.
 |
| | Fig. 8 (a) N2 adsorption/desorption isotherm (b) pore size distribution (c) 1/[Q(P/Po − 1)] vs. relative pressure (P/Po) plot of CuFe2O4 raw obtained at 623 K. | |
 |
| | Fig. 9 (a) N2 adsorption/desorption isotherm (b) pore size distribution (c) 1/[Q(P/Po − 1)] vs. relative pressure (P/Po) plot of CuFe2O4 acid obtained at 623 K. | |
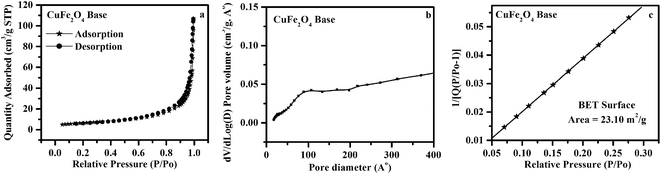 |
| | Fig. 10 (a) N2 adsorption/desorption isotherm (b) pore size distribution (c) 1/[Q(P/Po − 1)] vs. relative pressure (P/Po) plot of CuFe2O4 base obtained at 623 K. | |
Table 1 Williamson-Hall results and N2 adsorption/desorption isotherm measurement results of CuFe2O4 raw, acid and base obtained through BET surface area analyzer
| |
CuFe2O4 raw |
CuFe2O4 acid |
CuFe2O4 base |
| Crystallite size (WH) |
8.59 nm |
16.92 nm |
91.2 nm |
| Strain (WH) |
0.839% |
0.961% |
0.955% |
| Particle size DFT |
98.86 nm |
100.87 nm |
260 nm |
| Surface energy DFT |
219.37 m2 g−1 |
102.78 m2 g−1 |
59.95 m2 g−1 |
| Pore size BET |
15.38 nm |
18.57 nm |
28.08 nm |
| Total surface area |
60.68 m2 g−1 |
59.48 m2 g−1 |
23.10 m2 g−1 |
| t plot pore area |
2.02 m2 g−1 |
2.75 m2 g−1 |
1.42 m2 g−1 |
| t plot external surface area |
58.66 m2 g−1 |
56.73 m2 g−1 |
21.68 m2 g−1 |
| Pore volume |
0.233 cm3 g−1 |
0.276 cm3 g−1 |
0.162 cm3 g−1 |
3.7. Electrical properties
Fig. 11a–c show the impedance spectra of CuFe2O4 raw, acid and base samples obtained at 623 K and measured at 303 K, respectively. The real versus imaginary part of the impedance spectra showed two semicircles and the first semicircle at high frequency side corresponds to bulk or grain interior conduction and the second semicircle corresponds to the grain boundary conduction of the CuFe2O4 nanoparticles. The intercept of each depressed semicircle with real axis gives the bulk resistance (Rb) and grain boundary resistance (Rgb), which can be obtained from the fitted impedance data. The obtained impedance data were analyzed using Novacontrol, winfit software and the values of the circuit parameters were adjusted simultaneously for better fit. The best grain and grain boundary resistances and capacitances values are obtained from the fitted impedance data. The impedance spectra were effectively fitted with series combination of two parallel RC networks, consisting of grain interior resistance with grain interior capacitance and grain boundary resistance with grain boundary capacitance.28 The conductivity values were calculated by using the dimensions of the pellet and the respective resistance values obtained through fitting. The values were substituted in the formula σ = (t/A) × (1/R), where t is the thickness of the pellet and A is the area of the pellet. The electrical conduction in ferrites is due to the movement of electrons and holes, which are generated from the Fe2+ to Fe3+ and Cu2+ to Cu+ transitions.29–31 At low temperature, the probability of transition from Fe2+ to Fe3+ and Cu2+ to Cu+ is less because the wave functions of the adjacent ions are not overlapping. The ion diffusion through grain boundary will be more than interior, which is reflected in the obtained low resistance values for grain boundary than bulk; however, the presence of pores will reduce the conductivity.32–34 Based on the XRD and BET surface area measurement results, CuFe2O4 raw sample has a lower crystallite size and high surface area showing higher grain boundary conductivity than grain interior conductivity. CuFe2O4 acid samples have larger crystallite size and lesser surface area compared to CuFe2O4 raw samples. Moreover, the presence of large size pores in CuFe2O4 acid sample reduces the grain boundary conductivity. CuFe2O4 base sample have larger crystallite size and low surface area with large pore size. However, the presence of undecomposed carbon present on the boundary surface, as well as in the interior of CuFe2O4 base, increases the conductivity of both grain boundary and grain interior. The same results were also reflected in the FTIR results, in which impurity peaks are present in CuFe2O4 base sample calcined at 623 K.
 |
| | Fig. 11 Impedance spectra of CuFe2O4 (a) raw (b) acid (c) base samples obtained at 623 K and measured at 303 K. | |
3.8. Cyclic voltammogram
The CV profile shown in Fig. 12 corresponds to the changes between first and second cycles of battery fabricated using a mesoporous CuFe2O4 anode with PVdF and alginate binders. In Fig. 12, the irreversible reduction peaks observed around 0.7 V and 0.6 V moved to 0.89 V in the second cycle while using PVdF and alginate as binders. The observed peaks around 0.7 V and 0.6 V are attributed to Fe3+ to Fe0 reduction along with the solid electrolyte interface (SEI) formation. The observed anodic peaks observed at 1.64 and 1.88 V correspond to the oxidation of Fe0 to Fe3+ and Cu0 to Cu2+.35,36 The broad cathodic peak centered at 0.89 V observed in second cycle is attributed to the combined effect of Fe2O3 reduction and CuO reduction.37 Fig. 13a–d show the area covered under the oxidation and reduction cycles of 1st and 2nd cycles in the cyclic voltammetric curves for the battery fabricated using CuFe2O4 with alginate and PVdF as binders. The area under the curve reflects the total lithium ions and corresponding electrons involved in the electrochemical reaction.38 From Fig. 13a and b, the area covered under the initial discharge cycle of CuFe2O4 with alginate binder is greater than the area covered under the first oxidation cycle. This indicates that the lithium ions involved in the reduction (57.7%) of metal oxides along with the formation of Li2O, as well as for the formation of SEI, was more compared to the oxidation (42.3%) of corresponding metals. This is reflected in the initial discharge capacity as well as first charge capacity values of BCT results. In the second cycle, the area covered under the reduction cycle of CuFe2O4 with alginate binder is less than the area covered under the oxidation cycle. This indicates that the lithium ions involved in the initial discharge as well as first discharge during reduction (46.7%) comes back during oxidation (53.3%) process and the same is reflected in the charge/discharge capacity retention as well as high coulombic efficiency. The constant values of coulombic efficiency measured from charge/discharge cycle studies indicates that the lithium ions involved in the charge and discharge cycles are almost equal for the remaining cycles. This is attributed to better adhesive nature of alginate binder and corresponding electrical contact between electrode material with current collector and carbon black for longer cycles. However, the area covered under the initial discharge cycle of CuFe2O4 with PVdF binder is greater compared to the area covered under the oxidation curve, which is shown in Fig. 13c and d. This reflects that the initial usage of lithium ions during reduction (60.5%) of metal oxides along with the formation of Li2O and SEI formation is more compared to the oxidation (39.5%) process. However, during second cycle, the area covered under the reduction (55%) curve is more compared to the oxidation (45%). This indicates that the lithium ions involved in the reduction process are not returning back during oxidation. These results are reflected in the continuous decrement of charge/discharge capacity values and consequent reduction in columbic efficiency. The area covered under the reduction and oxidation cycles is more in CuFe2O4 with alginate binder compared to CuFe2O4 with PVdF binder, and this is due to the electrochemical bonding of alginate binder with electrode, which attracts more lithium ions for the reaction compared to CuFe2O4 with PVdF binder, where van der Waals attraction utilizes less lithium ions. From the ratio of the area covered under the reduction and oxidation reaction curves and the total area of full CV profile, the reduction percentage (60.5%) of CuFe2O4 with PVdF binder was more than the reduction percentage (57.7%) of PVdF with alginate binder. The oxidation percentage (39.5%) of CuFe2O4 with PVdF binder was less than the oxidation percentage (42.3%) of CuFe2O4 with alginate binder. This is due to the irreversible reaction of lithium with electrode in CuFe2O4 with PVdF binder. This is attributed to the poor adhesive nature of PVdF binder and consecutive side reactions, which are discussed in the charge/discharge measurements.
 |
| | Fig. 12 Cyclic voltammogram of CuFe2O4 raw with PVdF/Alginate binders. | |
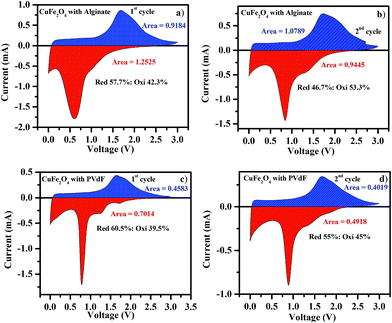 |
| | Fig. 13 Area covered under the cyclic voltammetry curves of CuFe2O4 raw with Alginate binder (a) 1st cycle (b) 2nd cycle and CuFe2O4 with PVdF binder (c) 1st cycle (d) 2nd cycle. | |
3.9. Charge/discharge measurements
Fig. 14a and b and 15 show the voltage profile vs. number of cycles and charge/discharge capacity vs. cycle number along with coulombic efficiency plots for the CuFe2O4 with PVdF and alginate binders. From Fig. 14a and 15, CuFe2O4 with PVdF binder electrode delivers a discharge capacity of 1461 mA h g−1 at first discharge. The observed first discharge capacity is higher than the theoretical capacity. This is attributed to the porous nature of anode material, as well as the formation of solid–electrolyte interface film, which consists of different organic/inorganic substances.39 The formed gel-like solid electrolyte interface decomposes during charging reaction and reforms during discharging reaction along with electron transfer. It increases the specific capacity of CuFe2O4 anode to a capacity higher than the theoretical capacity of CuFe2O4.40 A deep fall in the discharge capacity from the initial value to 412 mA h g−1 on 10th cycle is observed, and it comes down to 252 mA h g−1 after 20th cycle. After that, gradual capacity fading was observed up to 50th cycle. From Fig. 14b and 15, the battery fabricated using CuFe2O4 anode with alginate binder showing initial discharge capacity of 1300 mA h g−1. Here, the capacity fading is minimal and discharge capacity of 400 mA h g−1 is achieved even after the 50th cycle. The initial discharge capacity of CuFe2O4 with PVdF binder is higher compared to the initial discharge capacity of CuFe2O4 with alginate binder. The observed initial discharge capacity, greater than the theoretical capacity was due to the formation of SEI. The presence of alginate binder restricts the formation of SEI, as well as consecutive side reactions on further cycles, which is reflected in the decrement of initial discharge capacity in CuFe2O4 with alginate. The grain boundary surface and binders play important roles in the battery performance. The lithium ion diffusion at the grain boundary was more compared to the interior during charge and discharge reactions. The lithium ion diffusion length will be small and uniform for the particle with lower crystallite size and large grain boundary surface, which maintains uniform concentration gradient. Hence, the tensile stress occur on the surface of the materials due to lithium removal as well as Li2O formation during charging and discharging will be minimum.41 Nanoparticles with large grain boundary surface can avoid cracking due to tensile stress during charge and discharge reactions, and it maintains the capacity for longer cycles. On the other hand, the particle with large grain boundary surface having increased electrode electrolyte interface. The possibility of capacity degradation in the above material may be due to the increased side reactions.42 Hence, the particle with high grain boundary surface can minimize the capacity fading due to tensile stress and consecutive cracking; however, at the same time, the capacity fading due to increased side reaction with the electrolyte cannot be minimized. Mesoporous CuFe2O4 nanoparticles with larger grain boundary surface and carbon black are mixed with sodium alginate or PVdF binders to make the anode film. Sodium alginate is a copolymer of β-D-mannuronic acid (M) and α-L-guluronic acid (G) residues. The presence of COO− and OH− groups makes the polymer hydrophilic, and it is used as a thickening and gelling agent. Carboxylic groups present in the alginate binder easily form rigid, hydrogen bonds with oxidized surfaces, which enhance the stability and mechanical strength of the anode material. The rigid bonding of alginate binder minimizes the continuous electrolyte reaction with anode through SEI. Hence, restricted side reactions minimize the lithium ion loss, which maintains the charge/discharge capacity as well as coulombic efficiency. In the case of PVdF binder, lack of COO– groups make the binder a poor adhesive and it bonded with anode material through weak van der Waals bonding. The poor bonding of PVdF binder with anode material allows the electrolyte solution to react with the anode material continuously through SEI. The loss of lithium ions on each cycle reduces the charge/discharge capacity and showed coulombic efficiency reduction. Hence, the battery fabricated with alginate binder showed improved performance compared to PVdF binders upon formation and decomposition of Li2O during charging and discharge reactions. In order to determine the pore size and surface area effect on the battery performance, CuFe2O4 raw, acid and base samples calcined at 623 K were taken for battery fabrication with alginate binder. The fabricated batteries were cycled at a rate of 1 C, and the specific capacity vs. number of cycles for CuFe2O4 raw, acid and base samples with alginate binder is shown in Fig. S1 (ESI†). From Fig. S1,† the battery fabricated with CuFe2O4 raw sample shows high specific capacity with minimum capacity fading at high C rate compared to CuFe2O4 acid and base samples, and it was attributed to the high surface area and low crystallite size, which was reflected in BET and XRD results. The CuFe2O4 acid sample shows higher charge capacity than discharge capacity, which is attributed to the local acidity and may cause side reactions that increase the oxidation reaction.43 The CuFe2O4 base sample shows lower specific capacity but the capacity was retained for longer cycles. This was attributed to the lower surface area and high conducting contribution from undecomposed carbon present in the sample. Hence, CuFe2O4 raw sample with high surface area and low crystallite size showed better battery performance even at high C rates.
 |
| | Fig. 14 Voltage vs. specific capacity profile for (a) CuFe2O4 with PVdF binder (b) CuFe2O4 with alginate binder. | |
 |
| | Fig. 15 Specific capacity and coulombic efficiency vs. number of cycles for CuFe2O4 raw with PVdF and alginate binders. | |
4. Conclusions
Mesoporous copper ferrite nanoparticles were prepared successfully through citric acid and urea assisted combustion process. The local chemical modifications obtained through addition of acid/base to the synthesis process modified the combustion reaction and changed the crystallite size and surface area of CuFe2O4 nanoparticles. The characterization results confirmed the mesoporous nature of CuFe2O4 nanoparticles and lowest crystallite size and high surface area were obtained for CuFe2O4 raw sample. The electrical conductivity measurement through impedance spectroscopy showed the presence of grain boundaries and its high conducting contribution. CR 2032 coin cells were fabricated using mesoporous CuFe2O4 raw anode material with alginate/PVdF binders. The observed capacity fading was attributed to the side reaction of electrolyte and electrode material through high grain surfaces. The anode material fabricated using alginate binder minimized the capacity fading and improved the coulombic efficiency, which is attributed to the good adhesive nature of alginate binders that restrict the side reactions and minimize the lithium ion loss compared to PVdF binder. The battery performance of CuFe2O4 raw, acid and base samples with alginate binder cycled at a rate of 1 C showed that CuFe2O4 raw sample having high specific capacity and minimum capacity fading compared to CuFe2O4 acid and base sample, and this was attributed to the high surface area of CuFe2O4 raw sample.
Acknowledgements
NS gratefully acknowledges CSIR, DRDO, AICTE and DST, Govt. of India, for providing financial support in the form of major research projects. RI acknowledges UGC-RGNF for providing the JRF fellowship to complete her Ph.D. IP acknowledges TRR research grant offered by SASTRA University to perform this research. The authors also acknowledge CIF, Pondicherry University, for providing the SEM-EDS and DSC and BET surface area facilities.
Notes and references
- Z. H. Li, T. P. Zhao, X. Y. Zhan, D. S. Gao, Q. Z. Xiao and G. T. Lei, Electrochim. Acta, 2010, 55, 4594–4598 CrossRef CAS PubMed.
- J. Liu, Z. Guo, K. Zhu, W. Wang, C. Zhanga and X. Chen, J. Mater. Chem., 2011, 21, 11412–11417 RSC.
- Z. Zajickova, E. Rubi and F. Svec, J. Chromatogr. A, 2011, 1218, 3555–3558 CrossRef CAS PubMed.
- Y.-S. Hu, Y.-G. Guo, W. Sigle, S. Hore, P. Balaya and J. Maier, Nat. Mater., 2006, 5, 713–717 CrossRef CAS PubMed.
- Y. Zhang, S. Zha and M. Liu, Adv. Mater., 2005, 17, 487–491 CrossRef CAS PubMed.
- Y. Xiao, C. Hu and M. Cao, J. Power Sources, 2014, 247, 49–56 CrossRef CAS PubMed.
- R. K. Selvan, N. Kalaiselvi, C. O. Augustin, C. H. Doh and C. Sanjeeviraja, J. Power Sources, 2006, 157, 522–527 CrossRef PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- P. F. Teh, Y. Sharma, S. S. Pramana and M. Srinivasan, J. Mater. Chem., 2011, 21, 14999–15008 RSC.
- H. Zeng, T. Tao, Y. Wu, W. Qi, C. Kuang, S. Zhou and Y. Chen, RSC Adv., 2014, 4, 23145–23148 RSC.
- S. Permien, H. Hain, M. Scheuermann, S. Mangold, V. Mereacre, A. K. Powell, S. Indris, U. Schürmann, L. Kienle, V. Duppel, S. Harm and W. Bensch, RSC Adv., 2013, 3, 23001–23014 RSC.
- R. Sahay, P. S. Kumar, V. Aravindan, J. Sundaramurthy, W. C. Ling, S. G. Mhaisalkar, S. Ramakrishna and S. Madhavi, J. Phys. Chem. C, 2012, 116, 18087–18092 CAS.
- J. Vetter, P. Novák, M. R. Wagner, C. Veit, K. C. Möller, J. O. Besenhard, M. Winter, M. W. Mehrens, C. Vogler and A. Hammouche, J. Power Sources, 2005, 147, 269–281 CrossRef CAS PubMed.
- D. Wang, X. Wu, Z. Wang and L. Chen, J. Power Sources, 2005, 140, 125–128 CrossRef CAS PubMed.
- Z. Wang, S. Madhavi and X. W. (David) Lou, J. Phys. Chem. C, 2012, 116, 12508–12513 CAS.
- G. G. d'Ayala, M. Malinconico and P. Laurienzo, Molecules, 2008, 13, 2069–2106 CrossRef PubMed.
- Y. K. Jeong, T.-w. Kwon, I. Lee, T.-S. Kim, A. Coskun and J. W. Choi, Nano Lett., 2014, 14, 864–870 CrossRef CAS PubMed.
- A. M. Chockla, K. C. Klavetter, C. B. Mullins and B. A. Korgel, Chem. Mater., 2012, 24, 3738–3745 CrossRef CAS.
- J. Xu, S.-L. Chou, Q.-f. Gu, H.-K. Liu and S.-X. Dou, J. Power Sources, 2013, 225, 172–178 CrossRef CAS PubMed.
- X. Tao, R. Wu, Y. Xia, H. Huang, W. Chai, T. Feng, Y. Gan and W. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 3696–3702 CAS.
- S. Vivekanandhan, M. Venkateswarlu and N. Satyanarayana, J. Alloys Compd., 2007, 441, 284–290 CrossRef CAS PubMed.
- S. Vivekanandhan, M. Venkateswarlu and N. Satyanarayana, Mater. Chem. Phys., 2008, 109, 241–248 CrossRef CAS PubMed.
- I. Prakash, P. Muralidharan, N. Nallamuthu, M. Venkateswarlu and N. Satyanarayana, Mater. Res. Bull., 2007, 42, 1619–1624 CrossRef CAS PubMed.
- R. D. Waldron, Phys. Rev., 1955, 9, 1727–1735 CrossRef.
- L. Wang, W. Tang, Y. Jing, L. Su and Z. Zhou, ACS Appl. Mater. Interfaces, 2014, 6, 12346–12352 CAS.
- S. M. Yuan, J. X. Li, L. T. Yang, L. W. Su, L. Liu and Z. Zhou, ACS Appl. Mater. Interfaces, 2011, 3, 705–709 CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- H. L. Tuller, Solid State Ionics, 2000, 131, 143–157 CrossRef CAS.
- N. Ponpandian, P. Balaya and A. Narayanasamy, J. Phys.: Condens. Matter, 2002, 14(12), 3221–3238 CrossRef CAS.
- S. A. Mazen and A. M. El Taher, J. Alloys Compd., 2010, 498, 19–25 CrossRef CAS PubMed.
- S. A. Mazen and A. M. El Taher, Solid State Commun., 2010, 150, 1719–1724 CrossRef CAS PubMed.
- S. Chawla, M. Naraghi and A. Davoudi, Nanotechnology, 2013, 24(255708), 1–9 Search PubMed.
- J. M. Montes, F. G. Cuevas and J. Cintas, Appl. Phys. A, 2008, 92, 375–380 CrossRef CAS.
- W. Zhou, Y. Tang, R. Song, L. Jiang, K. S. Hui and K. N. Hui, Mater. Des., 2012, 37, 161–165 CrossRef CAS PubMed.
- Y. Ding, Y. Yang and H. Shao, Solid State Ionics, 2012, 217, 27–33 CrossRef CAS PubMed.
- Z. Xing, Z. Ju, J. Yang, H. Xu and Y. Qian, Electrochim. Acta, 2013, 102, 51–57 CrossRef CAS PubMed.
- L. Jin, Y. Qiu, H. Deng, W. Li, H. Li and S. Yang, Electrochim. Acta, 2011, 56, 9127–9132 CrossRef CAS PubMed.
- G. Kilibarda, D. V. Szabó, S. Schlabach, V. Winkler, M. Bruns and T. Hanemann, J. Power Sources, 2013, 233, 139–147 CrossRef CAS PubMed.
- A. Pan, Y. Wang, W. Xu, Z. Nie, S. Liang, Z. Nie, C. Wang, G. Cao and J.-G. Zhang, J. Power Sources, 2014, 255, 125–129 CrossRef CAS PubMed.
- L. Su, Y. Zhong and Z. Zhou, J. Mater. Chem. A, 2013, 1, 15158–15166 CAS.
- S. Han, J. Park, W. Lu and A. M. Sastry, J. Power Sources, 2013, 240, 155–167 CrossRef CAS PubMed.
- Y.-G. Guo, J.-S. Hu and L.-J. Wan, Adv. Mater., 2008, 20, 2878–2887 CrossRef CAS PubMed.
- S. H. Park, C. W. Moore, P. A. Kohl and J. Winnick, J. Electrochem. Soc., 2001, 148, A1346–A1351 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06673a |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid
citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea molar ratio as (M
urea molar ratio as (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U) = 1
U) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. The local chemical conditions of the combustion process were modified by adding excess acid or base. AR grade cupric nitrate trihydrate, ferric nitrate nanohydrate from Sigma Aldrich, India (99.9% purity), citric acid and urea from Fisher Scientific, India (99.9% purity) were used as precursor materials for the synthesis. The required cupric nitrate trihydrate (5 g) and ferric nitrate nonahydrate (16.9 g) were dissolved each in 100 ml distilled water and sonicated for 10 minutes in an ultrasonic probe type sonicator with a frequency of 40 kHz to obtain complete and homogeneous dissolution. Later, the mixture of cupric nitrate and ferric nitrate solutions were sonicated for 10 minutes and stirred at 353 K. The required citric acid (12 g) and urea (1.8 g) were mixed with 100 ml distilled water and added to the above solution under continuous stirring after 30 minutes, and the sample was labeled as CuFe2O4 raw. In another two sets of samples, 2 ml of concentrated nitric acid or 2 ml of ammonia solution was added in excess to the CuFe2O4 raw solution with continuous stirring and labeled as CuFe2O4 acid and CuFe2O4 base, respectively. CuFe2O4 raw, CuFe2O4 acid and CuFe2O4 base represent the sample preparation conditions of the combustion process and were maintained throughout the manuscript. Water evaporates from the solution mixture with continuous stirring at 353 K and polymeric resins were formed. The polymeric resins were dried in an oven at 333 K for 24 h and slow release of CO2 and ammonia gas vapors from the polymeric resin forms dried porous polymeric resins with volume expansion called polymeric intermediates. The addition of small quantities of acid and base changed the local chemical conditions and also changed the crystallinity of the byproduct formed in the polymeric intermediate. The volume expansion of the CuFe2O4 base PI was small compared to CuFe2O4 raw and acid PI. The polymeric intermediates were calcined at 443 and 623 K for 3 h under atmospheric conditions and mesoporous CuFe2O4 nanoparticles were obtained. The complete process was monitored using powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), scanning electron microscopy and energy dispersive X-ray spectrometry (SEM-EDS). The XRD spectra were obtained using a PANalytical XpertPRO diffractometer with Cu Kα radiation of wavelength 0.15418 nm from 10°–80° at a scan rate of 1.5° per minute. Fourier transform infrared spectra were recorded on a Shimadzu FTIR/8300/8700 spectrophotometer in the frequency range of 4000–400 cm−1 with a 2 cm−1 resolution for 20 scans. FTIR spectra were recorded using thin transparent pellets and each pellet was made by pressing an approximate mixture of 0.5 mg sample and 10 mg of spectra-pure KBr. The thermal behaviors of the prepared samples were analyzed using a TA Q20DSC differential scanning calorimeter under nitrogen gas flow. The particle size and morphology and elemental mapping of CuFe2O4 samples were analyzed using a Hitachi SN 3400N SEM and JEOL 6701 FESEM instruments. The FETEM images and selected area diffraction (SAED) pattern were taken using JEOL, JEM 2100F FETEM instrument. The pore size, surface area and pore size distribution were analyzed using Micromeritics GEMINI VII BET surface area analyzer. For impedance analysis, the samples were ground into fine powder and the samples were made into pellets of dimension 10 mm diameter and 1.5–2 mm thickness using a spectra Lab pelletizer by applying 5 ton pressure. The pellets were coated with silver paste for better electrical contact and maintained between silver electrodes for impedance measurement. The impedance measurements were made by applying an AC signal of 1 V with a frequency range from 1 Hz to 10 MHz using a Novacontrol Alpha AT high performance frequency response analyzer. The lithium ion battery electrodes were prepared by using active electrode material, carbon black, sodium alginate or PVdF as binders. The active material, carbon black and binders weight ratio was maintained at 70
0.5. The local chemical conditions of the combustion process were modified by adding excess acid or base. AR grade cupric nitrate trihydrate, ferric nitrate nanohydrate from Sigma Aldrich, India (99.9% purity), citric acid and urea from Fisher Scientific, India (99.9% purity) were used as precursor materials for the synthesis. The required cupric nitrate trihydrate (5 g) and ferric nitrate nonahydrate (16.9 g) were dissolved each in 100 ml distilled water and sonicated for 10 minutes in an ultrasonic probe type sonicator with a frequency of 40 kHz to obtain complete and homogeneous dissolution. Later, the mixture of cupric nitrate and ferric nitrate solutions were sonicated for 10 minutes and stirred at 353 K. The required citric acid (12 g) and urea (1.8 g) were mixed with 100 ml distilled water and added to the above solution under continuous stirring after 30 minutes, and the sample was labeled as CuFe2O4 raw. In another two sets of samples, 2 ml of concentrated nitric acid or 2 ml of ammonia solution was added in excess to the CuFe2O4 raw solution with continuous stirring and labeled as CuFe2O4 acid and CuFe2O4 base, respectively. CuFe2O4 raw, CuFe2O4 acid and CuFe2O4 base represent the sample preparation conditions of the combustion process and were maintained throughout the manuscript. Water evaporates from the solution mixture with continuous stirring at 353 K and polymeric resins were formed. The polymeric resins were dried in an oven at 333 K for 24 h and slow release of CO2 and ammonia gas vapors from the polymeric resin forms dried porous polymeric resins with volume expansion called polymeric intermediates. The addition of small quantities of acid and base changed the local chemical conditions and also changed the crystallinity of the byproduct formed in the polymeric intermediate. The volume expansion of the CuFe2O4 base PI was small compared to CuFe2O4 raw and acid PI. The polymeric intermediates were calcined at 443 and 623 K for 3 h under atmospheric conditions and mesoporous CuFe2O4 nanoparticles were obtained. The complete process was monitored using powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), scanning electron microscopy and energy dispersive X-ray spectrometry (SEM-EDS). The XRD spectra were obtained using a PANalytical XpertPRO diffractometer with Cu Kα radiation of wavelength 0.15418 nm from 10°–80° at a scan rate of 1.5° per minute. Fourier transform infrared spectra were recorded on a Shimadzu FTIR/8300/8700 spectrophotometer in the frequency range of 4000–400 cm−1 with a 2 cm−1 resolution for 20 scans. FTIR spectra were recorded using thin transparent pellets and each pellet was made by pressing an approximate mixture of 0.5 mg sample and 10 mg of spectra-pure KBr. The thermal behaviors of the prepared samples were analyzed using a TA Q20DSC differential scanning calorimeter under nitrogen gas flow. The particle size and morphology and elemental mapping of CuFe2O4 samples were analyzed using a Hitachi SN 3400N SEM and JEOL 6701 FESEM instruments. The FETEM images and selected area diffraction (SAED) pattern were taken using JEOL, JEM 2100F FETEM instrument. The pore size, surface area and pore size distribution were analyzed using Micromeritics GEMINI VII BET surface area analyzer. For impedance analysis, the samples were ground into fine powder and the samples were made into pellets of dimension 10 mm diameter and 1.5–2 mm thickness using a spectra Lab pelletizer by applying 5 ton pressure. The pellets were coated with silver paste for better electrical contact and maintained between silver electrodes for impedance measurement. The impedance measurements were made by applying an AC signal of 1 V with a frequency range from 1 Hz to 10 MHz using a Novacontrol Alpha AT high performance frequency response analyzer. The lithium ion battery electrodes were prepared by using active electrode material, carbon black, sodium alginate or PVdF as binders. The active material, carbon black and binders weight ratio was maintained at 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 for making electrode material slurries. Doubly distilled water was used as a solvent for making slurry using alginate binder, whereas N-methyl-2-pyrrolidone (NMP) solution was used for making slurry using PVdF binder. The coated slurries on a copper foil were dried and used as working electrodes, lithium foil was used as a reference electrode and Celgard polymer membrane was used as separator. 1 M LiPF6 dissolved in EC/DMC (1
10 for making electrode material slurries. Doubly distilled water was used as a solvent for making slurry using alginate binder, whereas N-methyl-2-pyrrolidone (NMP) solution was used for making slurry using PVdF binder. The coated slurries on a copper foil were dried and used as working electrodes, lithium foil was used as a reference electrode and Celgard polymer membrane was used as separator. 1 M LiPF6 dissolved in EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ratio was used as electrolyte. The CR 2032 coin cells were assembled in an argon filled VAC glove box using the prepared anode material. The cyclic voltammogram measurement was made for the prepared CR 2032 coin cells using CH instruments between 3 and 0.002 V at a scan rate of 0.2 mV s−1. The charge and discharge measurements were performed using a Bitrode battery life cycle tester between 3 V and 0.002 V at 0.1 C and 1 C rates.
1) ratio was used as electrolyte. The CR 2032 coin cells were assembled in an argon filled VAC glove box using the prepared anode material. The cyclic voltammogram measurement was made for the prepared CR 2032 coin cells using CH instruments between 3 and 0.002 V at a scan rate of 0.2 mV s−1. The charge and discharge measurements were performed using a Bitrode battery life cycle tester between 3 V and 0.002 V at 0.1 C and 1 C rates.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, which confirm the chelation of metal ions by citric acid. The band at 1725 cm−1 corresponds to the asymmetric vibrations of bridging COO− groups and the corresponding symmetric vibration observed at 1315 cm−1. Similar vibration bands are observed for CuFe2O4 acid and base PI samples. A band at 1384 cm−1 shows the presence of NO3− groups.22,23 The observed IR bands confirm the citric acid polymerization, chelation of metal ions and presence of nitrate and ammonia in the PI. The addition of excess acid and base in the reaction was reflected in the corresponding high intense vibration bands of nitrates and ammonia in the CuFe2O4 acid and base PI samples observed at 3173 and 1384 cm−1, respectively. Fig. 3b shows the FTIR spectra of CuFe2O4 raw, acid and base PI samples calcined at 623 K. During calcination, the organic impurities were decomposed from the sample, which is reflected in the absence of corresponding IR bands shown in Fig. 3b. The observed vibration bands at 590 and 414 cm−1 correspond to the octahedral and tetrahedral vibration of ferrites.24 By comparing the observed vibration bands of all CuFe2O4 samples calcined at 623 K, CuFe2O4 raw sample shows organic impurity free vibration bands corresponding to CuFe2O4. Hence, the FTIR results confirmed the purity of CuFe2O4 sample prepared using citric acid urea assisted combustion process.
O bonds, which confirm the chelation of metal ions by citric acid. The band at 1725 cm−1 corresponds to the asymmetric vibrations of bridging COO− groups and the corresponding symmetric vibration observed at 1315 cm−1. Similar vibration bands are observed for CuFe2O4 acid and base PI samples. A band at 1384 cm−1 shows the presence of NO3− groups.22,23 The observed IR bands confirm the citric acid polymerization, chelation of metal ions and presence of nitrate and ammonia in the PI. The addition of excess acid and base in the reaction was reflected in the corresponding high intense vibration bands of nitrates and ammonia in the CuFe2O4 acid and base PI samples observed at 3173 and 1384 cm−1, respectively. Fig. 3b shows the FTIR spectra of CuFe2O4 raw, acid and base PI samples calcined at 623 K. During calcination, the organic impurities were decomposed from the sample, which is reflected in the absence of corresponding IR bands shown in Fig. 3b. The observed vibration bands at 590 and 414 cm−1 correspond to the octahedral and tetrahedral vibration of ferrites.24 By comparing the observed vibration bands of all CuFe2O4 samples calcined at 623 K, CuFe2O4 raw sample shows organic impurity free vibration bands corresponding to CuFe2O4. Hence, the FTIR results confirmed the purity of CuFe2O4 sample prepared using citric acid urea assisted combustion process.














