Elucidation of the chemical composition of avian melanin†
Suet Yi Liua,
Matthew D. Shawkeyb,
Dilworth Parkinsonc,
Tyler P. Troya and
Musahid Ahmed*a
aChemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, USA. E-mail: mahmed@lbl.gov
bDepartment of Biology, University of Akron, Akron, OH, USA
cAdvanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA, USA
First published on 21st August 2014
Abstract
Our understanding of the chemical composition of melanin remains limited, due to a paucity of direct measurements. Avian feathers have an unparalleled diversity of melanin-based color mirroring their complex chemistry. Synchrotron-based photoionization mass spectrometry is used to determine the chemical composition of melanin from samples of black, brown, grey and iridescent feathers.
Melanin is the most pervasive and widespread pigment in living organisms and creates a broad range of black, brown and grey colors. Melanin possesses an intriguing set of physical and chemical properties including broadband monotonic absorbance, extremely low radiative quantum yield, and surprising condensed-phase electrical and photoconductive properties.1 This has led to the suggestion that melanin could be useful as a bio-inspired material in applications such as bioelectronics,2 chemical sensing, and photon detection.3 Avian feathers have an unparalleled diversity of melanin-based color that mirrors a diversity in their chemistry.4 The two chemical variants of integumentary melanin are black eumelanin and rusty red (rufous) pheomelanin. Both types are produced within organelles called melanosomes that are deposited directly from melanocytes into the developing integumentary structure (i.e. skin, hair or feather). While the morphology of melanosomes from feathers is statistically associated with their color5 and may play a role by means of scattering, it is their chemistry that predominantly determines their reflected color. A number of analytical techniques have been applied to decipher melanin's chemical structure,6 including mass spectrometry,7 the method used here. However, to the best of our knowledge, the results have thus far not provided for a definitive chemical characterization. These data are needed both to understand melanin's optoelectronic properties and to provide comparison points for melanin in fossil feathers. Because the two forms of melanin are chemically distinct as well as generally associated with distinct melanosome morphologies, we tested classifications of melanosomes through direct chemical measurement. This approach was undertaken with a set of samples representing the broad color categories produced by melanin: black, brown, grey, and iridescent. We hypothesized that each color would have a distinctive chemical composition, and specifically that black, iridescent and grey samples would be dominated by eumelanin, while brown samples would contain larger amounts of pheomelanin. We analyzed the chemical structures of melanin from a sample of feathers with melanin-based color using Laser Desorption Synchrotron Postionization (synchrotron-LDPI) mass spectrometry.8 A schematic of the experimental apparatus and an accompanying description may be found in the ESI.†
Fig. 1 shows typical mass spectra of extracted melanin from black, brown, and grey feathers measured by synchrotron-LDPI at 10.5 eV. Mass spectra for all 26 samples can be found in ESI, Fig. S7.† To aid in the classification and interpretation of these spectra, a statistical Peak Probability Contrast (PPC) analysis,9 was performed to determine which mass spectral peaks are most relevant. This allowed for grouping of significant mass peaks (peak signature) according to color. The process was to prepare a list of peaks from each mass spectrum for use as a training set. Once the statistical analysis has determined the signature for each group via training, it classifies color to percentage match in each data set to the gray, black, and brown mass spectral signatures. From this classification, chemical signatures may be deciphered for each color. (See ESI† for a more thorough description of PPC). The results of this analysis are presented in Fig. 2.
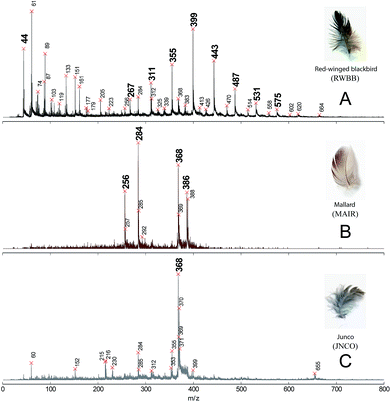 | ||
| Fig. 1 Mass spectra of extracted melanin from A: black, B: brown, and C: grey color of the extant feathers via synchrotron-LDPI at 10.5 eV. | ||
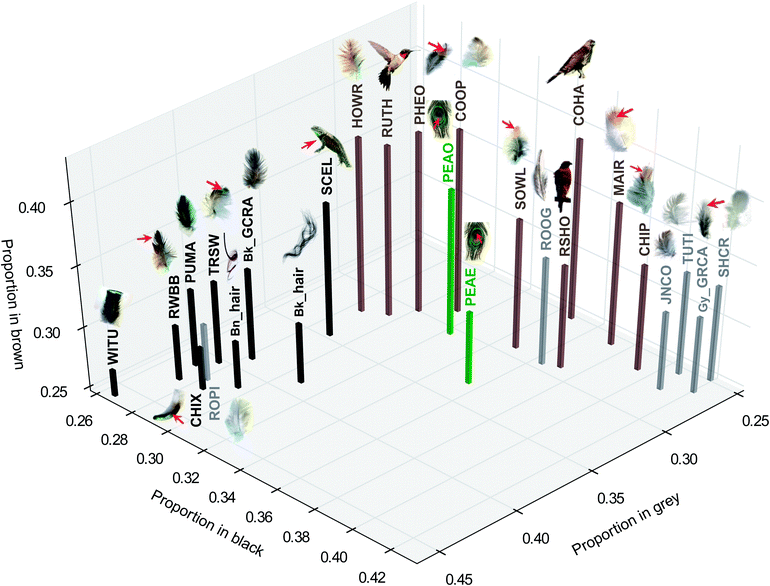 | ||
| Fig. 2 Peak probability contrasts (PPC) analysis projected onto three dimensions for black, brown, grey, and peacock (green) feathers. Where relevant, red arrows indicate the pigment extraction location. For abbreviations refer to Table S1 in †. | ||
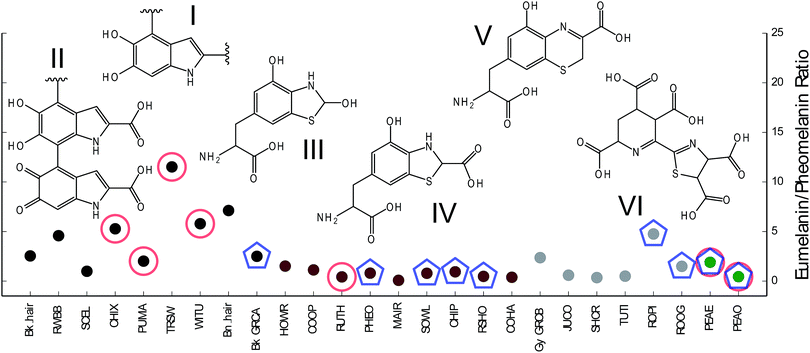
For black and iridescent feathers (wild turkey: WITU, domestic chicken Gallus domesticus: CHIX, red-winged blackbird Agelaius phoeniceus: RWBB, and purple martin Progne subis: PUMA), a spectral pattern representing the sequential loss of CO2 fragments was observed in the mass region between m/z 300–600. These peaks are emboldened in Fig. 1. The mass spectra of brown (Cooper's hawk Accipiter cooperii: COHA, COOP) and some iridescent feathers (e.g. Mallard Anas platyrhynchos: MAIR; and see ESI, Fig. S7†), are quite different: the most significant mass peaks m/z 256, 284, 368, and 386 are emboldened in Fig. 1B. The mass peak at m/z 284 corresponds to a benzothiazole-centered structure (see Fig. 3, structures III and IV). Peaks at m/z 312, and 340 correspond to conversion of the hydroxyl groups of benzothiazole to carboxylic groups. Evidence of these masses can be seen in COHA and COOP sample spectra (Fig. S7B†). There is also evidence for a benzothiazine-based structure (m/z 298, Fig. 3, structure V) in the brown feather mass spectrum, although it is much weaker than that for benzothiazole. Peaks at m/z 326 and 355 correspond to the conversion of one and two hydroxyl groups to carboxylic groups, respectively, while m/z 388 corresponds to two carboxylic groups adding to the structure shown in ESI, Fig. S6C.† For the mass spectra of grey feathers (ESI, Fig. S7C,† Junco: JNCO, Grey catbird: Gy_GCRB), the most pronounced peak is m/z 368 which could correspond to oxidized forms of benzothiazole. Interestingly, iridescent feathers from Mallard produced the cleanest spectrum for what can be construed as signatures for pheomelanin, while the mass spectra of other brown samples suggest that they also contain eumelanin. For instance, a spectral comb (separation of m/z 44 corresponding to COO) of m/z 223, 267, 311, 355, 399, 443, 487, and 531 is present in these mass spectra (ESI, Fig. S7B,† PHEO, CHIP, COOP). It has recently been suggested that pheomelanin could be composed of isoquinoline building blocks.10 Thiazolyl-pyridine pentacarboxylic acid (TPCA) was identified as a potential building block. This corresponds to m/z 381, (Fig. 3, VI) and upon saturation of the pyridine ring moiety, gives rise to m/z 386 and 388. This structure is what we interpret as giving rise to the strong signals in the brown mass spectra, and consider another signature of pheomelanin. Based on these characteristic peaks, we calculated the proportion of pheomelanin and eumelanin in each sample (see Fig. 3). While black colors are dominated by eumelanin, brown and grey contain higher proportions of pheomelanin. By contrast, iridescent colors vary from eumelanin- to pheomelanin-dominant. The highest proportional common peaks observed in black feathers, m/z 311, 399, 443 and 487 are most likely due to macromolecules composed of various redox forms of 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA), compounds which represent the building blocks of eumelanin, the fragments of which are depicted in Fig. 3, I and II. m/z 388 can arise from two carboxylic acid groups being added to the benzothiazine derivative (m/z 298 + 90). For grey feathers, the highest proportional common peaks are m/z 267, 298 and 368. Note that most of the peaks observed in the grey color training sets are also common to black and brown color sets, hence the common peak list of grey color is not as distinct compared to the other two colors. This suggests that grey color, like iridescence, can be produced by a wider variety of chemistries other than black or brown.
The PPC analysis9 examining the relationship between integumentary color and the molecular structure of melanin reveals broadly similar patterns to the analyses of eu- and pheomelanin (see ESI† and Fig. 2 and 3). While black melanin is well separated from brown and grey, brown and grey group closely together. Most samples were correctly predicted. For example, the black feather from the head of a grey catbird (Bk_GCRB) is predicted as black, while the grey feather from the body of the same species is predicted as grey. The eu-pheomelanin ratios of these two samples are very similar (Fig. 3), suggesting that additional chemistries may also contribute to color differences. Interestingly, the feather from the outer brownish part of the ring of peacock feather “eye” (PEAO) was predicted as brown, while the blue portion (PEAE) fell outside the range of any other color, suggesting extensive chemical modification. However, the other two grey test samples were misclassified as either brown or black, again indicating the variability of this color. In summary, our analyses suggest that black color in avian melanin originates from eumelanin (clusters of oxidized forms of DHI and DHICA). Brown color is composed mostly of pheomelanin (composed of oxidized versions of benzothiazine, benzothiazole, and isoquinolines) with also various contributions from eumelanin. Finally grey color is derived mostly from pheomelanic building blocks with minimal contributions from eumelanin, and isoquinoline derivatives. Avian melanin-based color thus appears to be more chemically complex than that of mammalian hair in which, for example, grey color is caused by a low dose of eumelanin and iridescent color is absent. This enhanced complexity mirrors the enhanced diversity of avian colors relative to those in mammals. Whether this complexity facilitated or was driven by the evolution of highly-visual communication in birds remains to be seen.
A number of experimental and theoretical studies have taken a molecular electronic structure approach to the chemical composition of melanin.11,12 The general consensus is of a ‘chemical disorder’ model where different chemical monomers may cross link and connect through various sites to provide a structure.13 Electronic structure calculations suggest that such a model could give rise to the broadband non-structured absorption that is typically seen in melanin derived synthetically or from human hair and squid ink sacs. While the monomer spectrum for DHICA is centered at ∼300 nm, (see ESI, Fig. S10†) calculations on dimers and oligomers performed by other groups suggest strong red shifts in the absorption spectra.11,13 A superposition of these spectra could give rise to the measured absorption spectra. Our results for black feathers broadly support this view. While there are subtle differences in the mass spectra, broadly it would appear that a DHICA/DHI-based oligomer structure would confer black as seen in the clustering on the left in Fig. 2. As shown in this work and by others, an increase in pheomelanin content would lead to a shift to brown. Electronic structure calculations generating an absorption spectrum were performed on m/z 193, 282, 284, 298 and 384 which are representative of DHICA, benzothiazole, benzothiazine, and isoquinoline monomer units, respectively. Pursuant readers can see the simulated spectra in ESI, Fig. S10.† Upon addition of sulfur to the ring, there is a lowering of the absorption cross-section, especially in the case of the benzothiazole unit (m/z 284). Cross-linking and oligomerization of these units could lead to a shift in the overall absorption pattern. This is again borne out in our experimental results where, brown is clustered to the right in Fig. 2, suggesting that distinct chemical moieties confer color to melanin. It is interesting to note that recent photoemission studies of dark brown and blue-green human irides report that the UV absorption cross-section coefficient of melanosomes decreases with increasing pheomelanin content.14 Finally, recently various chemical methods to detect melanin in fossilized animals have relied on single comparisons to eumelanin and pheomelanin standards.15,16 Our more intensive sampling approach that incorporates mixtures of pheomelanin and eumelanin as they are found in birds may enable more precise predictions of color and hence greater inference on the role of color in the ecology and evolution of animals in deep time.
Conclusions
Synchrotron based photoionization mass spectrometry led to the chemical characterization of melanin extracted from bird feathers. Color is correlated to chemical structure and provides insight towards the electronic structure of melanin.Acknowledgements
The authors (MA, SYL, DP, TPT), the Advanced Light Source and the National Energy Research Scientific Computing Center are supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract no. DE-AC02-05CH11231. MDS is supported by AFOSR grant FA9550-09-1-0159, HFSP grant RGY0083 and NSF grant EAR-1251895.References
- J. D. Simon, D. Peles, K. Wakamatsu and S. Ito, Pigm. Cell Melanoma Res., 2009, 22, 563–579 CrossRef CAS PubMed.
- M. d'Ischia, A. Napolitano, A. Pezzella, P. Meredith and T. Sarna, Angew. Chem., Int. Ed., 2009, 48, 3914–3921 CrossRef PubMed.
- A. B. Mostert, B. J. Powell, F. L. Pratt, G. R. Hanson, T. Sarna, I. R. Gentle and P. Meredith, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 8943–8947 CrossRef CAS PubMed.
- K. J. McGraw, R. J. Safran and K. Wakamatsu, Funct. Ecol., 2005, 19, 816–821 CrossRef PubMed.
- Q. G. Li, K. Q. Gao, Q. J. Meng, J. A. Clarke, M. D. Shawkey, L. D'Alba, R. Pei, M. Ellison, M. A. Norell and J. Vinther, Science, 2012, 335, 1215–1219 CrossRef CAS PubMed.
- J. D. Simon and D. N. Peles, Acc. Chem. Res., 2010, 43, 1452–1460 CrossRef CAS PubMed.
- A. Dzierzega-Lecznar, S. Kurkiewicz and K. Stepien, J. Mass Spectrom., 2012, 47, 242–245 CrossRef CAS PubMed.
- O. Kostko, L. K. Takahashi and M. Ahmed, Chem.–Asian J., 2011, 6, 3066–3076 CrossRef CAS PubMed.
- R. Tibshirani, T. Hastie, B. Narasimhan, S. Soltys, G. Y. Shi, A. Koong and Q. T. Le, Bioinformatics, 2004, 20, 3034–3044 CrossRef CAS PubMed.
- A. Napolitano, L. Panzella, L. Leone and M. D'Ischia, Acc. Chem. Res., 2013, 46, 519–528 CrossRef CAS PubMed.
- P. Meredith, B. J. Powell, J. Riesz, S. P. Nighswander-Rempel, M. R. Pederson and E. G. Moore, Soft Matter, 2006, 2, 37–44 RSC.
- B. J. Powell, T. Baruah, N. Bernstein, K. Brake, R. H. McKenzie, P. Meredith and M. R. Pederson, J. Chem. Phys., 2004, 120, 8608–8615 CrossRef CAS PubMed.
- E. Kaxiras, A. Tsolakidis, G. Zonios and S. Meng, Phys. Rev. Lett., 2006, 97, 218102 CrossRef.
- D. N. Peles and J. D. Simon, J. Phys. Chem. B, 2010, 114, 9677–9683 CrossRef CAS PubMed.
- J. Lindgren, P. Uvdal, P. Sjovall, D. E. Nilsson, A. Engdahl, B. P. Schultz and V. Thiel, Nat. Commun., 2012, 3, 824 CrossRef PubMed.
- K. Glass, S. Ito, P. R. Wilby, T. Sota, A. Nakamura, C. R. Bowers, J. Vinther, S. Dutta, R. Summons, D. E. G. Briggs, K. Wakamatsu and J. D. Simon, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 10218–10223 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Description of experimental procedure, mass spectra, laser & synchrotron energy dependence, statistical analysis, calculated absorption spectra of monomers. See DOI: 10.1039/c4ra06606e |
| This journal is © The Royal Society of Chemistry 2014 |