A colorimetric and near-infrared fluorescent probe for biothiols and its application in living cells†
Abstract
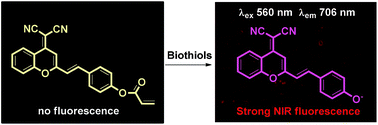
A new near-infrared (NIR) fluorescent probe, which contains a conjugated dicyanomethylene-benzopyran structure as the NIR fluorophore and an acrylate moiety as the reaction site, was found to be a promising NIR probe for biothiols (Cys, Hcy and GSH). This probe shows rapid response, high selectivity and sensitivity for biothiols (particularly for Cys and Hcy), accompanied by distinct color changes and significant NIR fluorescence turn on responses. The detection limit for Cys was found to be 81 nM. In addition, the imaging of biothiols by this probe was also successfully applied in living cells, indicating that this probe holds promise for applications in biological samples.

 Please wait while we load your content...
Please wait while we load your content...