The construction of a fluorescent nano-probe and its application in detecting transgenic Bt rice TT51-1
Guangyuan Zhanga,
Hongwei Suna,
Fan Lia,
Shuke Yanga,
Xiao Hui Xua,
Rui Gaoa,
Lei Zhao*b and
Xingbo Lu*a
aInstitute of Plant Protection, Shandong Academy of Agricultural Sciences, Shandong Key Laboratory of Plant Virology, 202 North Industrial Road, Jinan, China. E-mail: zhanggyforever@sina.com; Tel: +86-0531-83179095
bCollege of Life Science, Shandong Normal University, 88 East Wenhua Road, Jinan, China. E-mail: zhaolei@sdu.edu.cn
First published on 28th August 2014
Abstract
In this study, we used 13 nm gold nanoparticles (AuNPs) as the quenching group and Cy3/Cy5 fluorescent dye as the reporting group to develop a non-PCR method to detect transgenic rice TT51-1. The results showed that it had high sensitivity and specificity, and was time-efficient, inexpensive and simple to use.
According to a report by the International Service for the Acquisition of Agri-biotech Applications (ISAAA), the global growth area of transgenic crops reached 175.2 million hectares in 2013, an increase of 3%, or 5 million hectares, from the year 2012 (ref. 1). Rice is one of the main food crops of human beings. Since the cultivation of the first transgenic rice by Toriyama in 1988, it has always been a hot topic in modern agricultural biotechnology research. Because of the potential risks of transgenic crops, over 50 countries globally require labeling for transgenic food and feed, and have developed related regulations on the contents of transgenic components.2 TT51-1 is an insect-resistant transgenic rice event harboring a hybrid Cry1Ab/Ac gene driven by the rice actin1 gene promoter and the nopaline synthase (NOS) terminator.3 The current detection method for TT51-1 is PCR.4 However, it is inevitable during PCR procedures to encounter situations where the amplification has low efficiency or fails altogether, the amplification product contains non-specific components, or incorrect nucleotide pairing occurs. In this study, we used 13 nm gold nanoparticles as the quenching group and developed a direct and rapid detection method to detect the rice endogenous housekeeping gene SPS and the exogenous Bt gene (Cry1Ab/Ac) of transgenic rice TT51-1.
Gold nanoparticles (AuNPs) are minute particles with a diameter range of 1–100 nm. As they exist as colloids in aqueous solutions, they are normally referred to as colloidal gold in biological research. As compared to organic dye,5 the special properties and functional surface of gold nanoparticles enable them to act as multi-mode assembly platforms for the free assembly of oligonucleotides,6 antibodies,7 proteins8 and magnetic material9 so they can be used in the exploration and research of biological systems. The integration of gold nanoparticles and nucleic acids, proteins, biological macromolecules, drugs and other biological molecules has the advantages of easy preparation, stable chemical properties, good biocompatibility, simplicity of use and low cost, and has been widely used in biological molecule detection.10 As far as we know, there have been no reports on the use of fluorescent nano-probes in transgenic plant detection.
The DNA sequences used in this study are listed in Table 1. The recognition chains contained 30 nucleotides, including 21 nucleotides that were complementary to the target mRNA (Cry1Ab/Ac: EU880444.1; SPS: U33175.1); nine repeated A nucleotides at the thio-group end were used as a spacer. Potential hairpin structures in the DNA molecules were predicted using the Structure 2.3.4 software. The signal chains were complementary to the recognition chains. The DNA molecules were synthesized by Sangon Biotech Co., Ltd., Shanghai, China and were purified using HPLC. Thio-modified DNA was reduced using tris-(2-carboxyethyl) phosphine hydrochloride (TCEP·HCl) before the conjugation reaction with gold nanoparticles.
| Oligonucleotide | Sequence |
|---|---|
| SPS recognition chains | 5′-CCTGTGCTTTATCCATAGACT(A)9-(CH2)6-SH-3′ |
| SPS signal chains | 5′-Cy3-AGTCTATGGA-3′ |
| Cry1Ab/Ac recognition chains | 5′-TGCTGTTGAGTCTAACGAGGT(A)9-(CH2)6-SH-3′ |
| Cry1Ab/Ac signal chains | 5′-Cy5-ACCTCGTTAG-3′ |
The two thiolated oligoribonucleotide chains (10 μM of each) were separately mixed in phosphate buffer solution (PBS; 137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH 7.4) at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
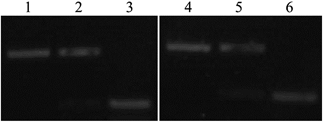
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 with their complementarily matched short chains, which were modified by fluorescent dyes (signal chains). The mixtures were heated to 75 °C and kept at this temperature for 10 min, before being slowly cooled to room temperature. They were left standing in the dark for 12 h to enable complete hybridization of the two double-stranded chains. PAGE was used to verify that the hybridization was successful. And results are shown in Fig. 1. Based on the differences in molecular weights, bands appeared in different positions in the lanes. Because of their increased molecular weights, the hybridization products in the middle lane showed a band at the top, while underneath this was a band of lower molecular weight, which represented excess signal chain as the hybridization ratio of the two single-stranded chains was 1
1.2 with their complementarily matched short chains, which were modified by fluorescent dyes (signal chains). The mixtures were heated to 75 °C and kept at this temperature for 10 min, before being slowly cooled to room temperature. They were left standing in the dark for 12 h to enable complete hybridization of the two double-stranded chains. PAGE was used to verify that the hybridization was successful. And results are shown in Fig. 1. Based on the differences in molecular weights, bands appeared in different positions in the lanes. Because of their increased molecular weights, the hybridization products in the middle lane showed a band at the top, while underneath this was a band of lower molecular weight, which represented excess signal chain as the hybridization ratio of the two single-stranded chains was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2. Thus, the two single-stranded DNA chains were successfully hybridized by annealing.
1.2. Thus, the two single-stranded DNA chains were successfully hybridized by annealing.
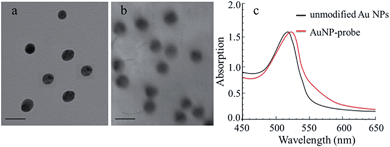
Thirteen-nanometer AuNPs at 3.5 nm were produced according to Natan's method of reducing chlorauric acid by sodium citrate.11 One-hundred-milliliters of 0.01% (m m−1) HAuCl4 aqueous solution was heated to boiling, and 3.5 mL of 1% (m m−1) sodium citrate aqueous solution was quickly added with vigorous stirring. The solution turned from light yellow to colorless and then gradually to wine-red. It was kept boiling at the end for 10 min. The heat was then turned off and the solution was stirred for another 15 min and slowly cooled to room temperature. The concentration of the AuNPs was then increased to 17 nM by centrifugation (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 × g, 20 min, 4 °C) to improve their salt-resistance and shelf-life.12 The prepared AuNP solution was filtered using a 0.22 μm membrane and stored at 4 °C. The shapes, sizes and optical properties of the AuNPs were analyzed using transmission electron microscopy (TEM, JEM-100CX II, JEOL, Japan) and Nano-Drop 1000 UV-vis spectrophotometer, respectively. A TEM picture of the AuNPs obtained by the sodium citrate reduction method is shown in Fig. 2a. The AuNPs showed good dispersion and even diameter distribution. The normalized UV-visible absorption spectrum indicated that the AuNPs had a maximum plasmon resonance absorption peak at 519 nm, which was consistent with the characteristic absorption peak of 13 nm AuNPs, as shown in Fig. 2c.
930 × g, 20 min, 4 °C) to improve their salt-resistance and shelf-life.12 The prepared AuNP solution was filtered using a 0.22 μm membrane and stored at 4 °C. The shapes, sizes and optical properties of the AuNPs were analyzed using transmission electron microscopy (TEM, JEM-100CX II, JEOL, Japan) and Nano-Drop 1000 UV-vis spectrophotometer, respectively. A TEM picture of the AuNPs obtained by the sodium citrate reduction method is shown in Fig. 2a. The AuNPs showed good dispersion and even diameter distribution. The normalized UV-visible absorption spectrum indicated that the AuNPs had a maximum plasmon resonance absorption peak at 519 nm, which was consistent with the characteristic absorption peak of 13 nm AuNPs, as shown in Fig. 2c.
Equal amounts of double-stranded DNA molecules that were separately labeled with Cy3 and Cy5 fluorescent dyes were mixed and added to an AuNP solution (17 nM), making the final concentration of AuNPs 10 nM. After the mixture was slowly stirred for 20 min, a solution of 1 M PBS (100 mM PB, pH 7.0, 1 M NaCl) was gradually added until the salt concentration reached 0.1 M. Stirring was continued for 16 h before the reaction was terminated. Then, the reaction solution was centrifuged at high speed (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 × g, 20 min) and the precipitate was re-dispersed in 100 μL of 0.1 M PBS. This process was repeated three times to completely separate the probes from the reagents. The probes in the final dispersion were further purified by filtering through a membrane with a 0.22 μm pore diameter. By reacting with the modifying thio groups of the DNA, the AuNPs formed covalent Au–S bonds and Au-DNA secondary structure. We used TEM and the UV-visible absorption spectrum to characterize the produced DNA-AuNP probe, as shown in Fig. 2b and c. Compared with un-modified AuNPs, TEM showed that, after double-stranded DNA modification, the edges of the AuNPs became blurred and their diameter increased, while dispersion remained good. The normalized UV-visible absorption spectrum showed that after the DNA was assembled onto the AuNPs, the maximum characteristic absorption peak shifted from 519 nm to 524 nm; this was because the diameter of the DNA-AuNP probes increased compared with the AuNPs.
930 × g, 20 min) and the precipitate was re-dispersed in 100 μL of 0.1 M PBS. This process was repeated three times to completely separate the probes from the reagents. The probes in the final dispersion were further purified by filtering through a membrane with a 0.22 μm pore diameter. By reacting with the modifying thio groups of the DNA, the AuNPs formed covalent Au–S bonds and Au-DNA secondary structure. We used TEM and the UV-visible absorption spectrum to characterize the produced DNA-AuNP probe, as shown in Fig. 2b and c. Compared with un-modified AuNPs, TEM showed that, after double-stranded DNA modification, the edges of the AuNPs became blurred and their diameter increased, while dispersion remained good. The normalized UV-visible absorption spectrum showed that after the DNA was assembled onto the AuNPs, the maximum characteristic absorption peak shifted from 519 nm to 524 nm; this was because the diameter of the DNA-AuNP probes increased compared with the AuNPs.
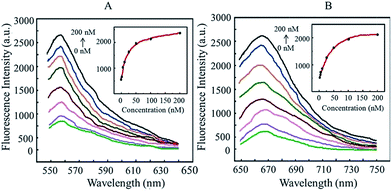
The synthesized DNA-AuNP probe was diluted to 1 nM with buffer (10 mM PBS, pH 7.4, 100 mM NaCl, 1 mM MgCl2) and incubated with the mRNA of transgenic Bt rice TT51-1 at 42 °C for 1 h. Changes in fluorescent signals were recorded by a fluorometer (Edinburgh Instruments Ltd, UK). In the sensitivity test, the probes were separately incubated with TT51-1 mRNA at 0, 2, 5, 10, 25, 50, 100 and 200 nM for 1 h (42 °C, 1 mL total mixture volume) and the corresponding fluorescent signals were collected. Cy3-labeled SPS detection signals were recorded as the fluorescence intensity at a wavelength of 560 nm (signal collection range: 550–640 nm); Cy5-labeled Bt detection signals were recorded as the fluorescence intensity at a wavelength of 668 nm (signal collection range: 650–750 nm). All tests were repeated three times and each repeat used three parallels. Without target molecules, the fluorescent dye molecules near the AuNP surface were quenched and gave no fluorescent signal. In the presence of target TT51-1 mRNA, because there were more target nucleotides than the 10 nucleotides in the “signal” chain, the complementary pairing of the recognition sequence was easier and out-competed the “signal” short chain that was previously hybridized with the recognition sequence. Thus, the Cy3/Cy5 fluorescent signals were restored, allowing simultaneous detection of the two target molecules. The results of hybridization between the DNA-AuNP probes we built and TT51-1 mRNA is shown in Fig. 3. In the experiment, we mixed and incubated the DNA-AuNP probe with various amounts of TT51-1 mRNA at a final concentration of 1 nM, and then detected the changes in the corresponding Cy3 and Cy5 fluorescent signals of the SPS and Cry1Ab/Ac genes. As shown in Fig. 3, with increasing target mRNA concentrations (from 0 to 200 nM), the corresponding fluorescent signal intensity gradually increased. The experimental results showed that the detected fluorescence intensity was dependent on target molecule concentration. The target DNA molecules competed with the signal chains for complementary pairing, resulting in the release of signal chains modified by Cy3 and Cy5, enabling the detection of the corresponding restored fluorescent signals.
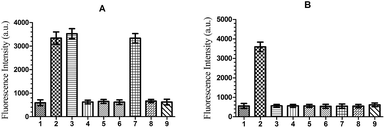
The DNA-AuNP probe (1 nM) was incubated separately with the mRNA (200 nM) of transgenic Bt rice TT51-1, non-transgenic Bt rice LLrice62, non-transgenic Bt maize MIR162 and NK603, non-transgenic Bt soybean MON87705 and non-transgenic rice, maize and soybean. The reactions were conducted in 1 mL hybridization buffer and the corresponding fluorescence signals were collected. All tests were repeated three times and each repeat used three parallels. The results are shown in Fig. 4. The hybridization between the DNA-AuNP probes and the endogenous housekeeping gene SPS (TT51-1, LLrice62 and non-genetically modified rice), and between the DNA-AuNP probe and the exogenous Cry1Ab/Ac gene (TT51-1) produced very strong fluorescent signals, while no obvious signal was produced in the other samples. These data proved that the DNA-AuNP probe we constructed was specific to transgenic Bt rice TT51-1.
Regarding PCR methods for TT51-1 detection,4 the amplification of specific DNA sequences is often compromised by various issues such as amplification of undesired sequences, low yield, primer self-ligation and self-amplification, or even complete amplification failure (no product). In traditional PCR, agarose gel electrophoresis cannot distinguish proximal products effectively, and the ethidium bromide used in agarose gels has potential carcinogenicity. For real-time PCR, the expensive reagents (fluorescent dye) and Taqman probes lead to a high cost of examination. Additionally, the PCR cycles required prolong the detection period. Compared with PCR, fluorescent nano-probes have advantages such as high accuracy, less time usage and low cost.
Materials and RNA sample extraction
Transgenic Bt rice TT51-1 was provided by the Center of Science and Technology Development, Ministry of Agriculture of the People's Republic of China (Beijing, China). Non-transgenic Bt rice LLrice62, non-transgenic Bt maize MIR162 and NK603, and non-transgenic Bt soybean MON87705 are maintained at our laboratory. Non-transgenic rice, maize and soybean were purchased from a local farmers' market. RNA was extracted from experimental samples using a RNA extraction kit (TaKaRa MiniBEST Plant RNA Extraction Kit, TaKaRa, Dalian, China). The purity and concentration of the extracted templates were measured using a Nano-Drop 1000 UV-vis spectrophotometer (NanoDrop, Wilmington, DE, USA).Conclusions
The integration of nano-materials and biotechnology has become an important frontline in the development of modern biochemical analysis tools. In this study, we constructed a fluorescent nano-probe that could simultaneously detect the rice endogenous housekeeping gene SPS and the exogenous Bt gene (Cry1Ab/Ac), and successfully applied these probes in the direct and rapid detection of transgenic Bt rice TT51-1. However, the quantitation of transgenic products using the fluorescent nano-probe needs further research.Notes and references
- C. James, 2013, ISAAA Report on Global Status of Biotech/GM Crops, http://www.isaaa.org Search PubMed.
- Ministry of Agriculture and Forestry of South Korea. Guidelines for Labeling of Genetically Modified Agricultural Products; MAF Notification, 31, 2000; European Commission. Commission Regulation (EC) no. 1829/2003 of September 22, 2003, concerning genetically modified food and feed. Official J. Eur. Communities 2003a, L268, 1–23; European Commission Regulation (EC) no. 1830/2003 of September 22, 2003, concerning the traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC. Official J. Eur. Communities 2003b, L268, 24–28; Matsuoka, T. GMO Labeling and Detection Methods in Japan; APEC-JIRCAS Joint Symposium and Workshop on Agricultural Biotechnology, 2001; Order 10; Ministry of Agriculture of the People's Republic of China, Beijing, China, 2002.
- J. Tu, K. Datta, M. Alam, Y. Fan, G. Khush and S. Datta, Plant Biotechnol., 1998, 15, 195–203 CrossRef CAS; J. Tu, G. Zhang, K. Datta, C. Xu, Y. He, Q. Zhang, K. Gurdev Singh and S. Datta, Nat. Biotechnol., 2000, 18, 1101–1104 CrossRef PubMed; J. Tu, K. Datta, N. Oliva, G. Zhang, C. Xu, G. Khush, Q. Zhang and S. Datta, Plant Biotechnol., 2003, 1, 155–165 CrossRef PubMed.
- Y. H. Wu, L. T. Yang, Y. L. Cao, G. W. Song, P. Shen, D. B. Zhang and G. Wu, J. Agric. Food Chem., 2013, 61, 5953–5960 CrossRef CAS PubMed; G. Wu, Y. H. Wu, S. J. Nie, L. Zhang, L. Xiao, Y. l. Cao and C. M. Lu, Food Chem., 2010, 119, 417–422 CrossRef PubMed.
- (a) Z. Khan, T. R. Chetia, A. K. Vardhaman, D. Barpuzary, C. V. Sastri and M. Qureshi, RSC Adv., 2012, 2, 12122–12128 RSC; (b) J. A. Lines, Z. Q. Yu, L. M. Dedkova and S. X. Chen, Biochem. Biophys. Res. Commun., 2014, 443, 308–312 CrossRef CAS PubMed; (c) T. Kabashima, Z. Yu, C. Tang, Y. Nakagawa, K. Okumura, T. Shibata, Z. Lu and M. Kai, Peptides, 2008, 29, 356–363 CrossRef CAS PubMed; (d) Z. Q. Yu, R. M. Schmaltz, T. C. Bozeman, R. Paul, M. J. Rishel, K. S. Tsosie and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 2883–2886 CrossRef CAS PubMed; (e) C. Bhattacharya, Z. Q. Yu, M. J. Rishel and S. M. Hecht, Biochemistry, 2014, 53, 3264–3266 CrossRef CAS PubMed; (f) C. Fu, R. Xia, T. Zhang, Y. Lu, S. Zhang, Z. Yu, T. Jin and X. Mou, PLoS One, 2014, 9, e90446 Search PubMed; (g) Z. Yu, T. Kabashima, C. Tang, T. Shibata, K. Kitazato, N. Kobayashi, M. K. Lee and M. Kai, Anal. Biochem., 2010, 397, 197–201 CrossRef CAS PubMed.
- S. T. Guo, Y. Y. Huang, Q. Jiang, Y. Sun, L. D. Deng, Z. C. Liang, Q. Du, J. F. Xing, Y. L. Zhao, P. C. Wang, A. J. Dong and X. J. Liang, ACS Nano, 2010, 4, 5505–5511 CrossRef CAS PubMed; Z. Y. Xiao, C. W. Ji, J. J. Shi, E. M. Pridgen, J. Frieder, J. Wu and O. C. Farokhzad, Angew. Chem., Int. Ed., 2012, 47, 11853–11857 CrossRef PubMed.
- D. X. Li, Q. He, Y. Cui, L. Duan and J. B. Li, Biochem. Biophys. Res. Commun., 2007, 355, 488–493 CrossRef CAS PubMed.
- L. Calzolai, F. Franchini, D. Gilliland and F. Rossi, Nano Lett., 2010, 10, 3101–3105 CrossRef CAS PubMed.
- J. Zhu, Y. Lu, Y. Li, J. Jiang, L. Cheng, Z. Liu, L. Guo, Y. Pan and H. Gu, Nanoscale, 2014, 6, 199–202 RSC.
- (a) R. Wilson, Chem. Soc. Rev., 2008, 37, 2028–2045 RSC; (b) B. Dubertret, M. Calame and A. J. Libchaber, Nat. Biotechnol., 2001, 19, 365–370 CrossRef CAS PubMed; (c) R. A. Sperling, P. R. Gil, F. Zhang, M. Zanella and W. J. Parak, Chem. Soc. Rev., 2008, 37, 1896–1908 RSC.
- K. C. Grabar, R. G. Freeman, M. B. Hommer and M. J. Natan, Anal. Chem., 1995, 67, 735–743 CrossRef CAS.
- Z. Q Liang, J. Zhang, L. H. Wang, S. P. Song, C. H. Fan and G. X. Li, Int. J. Mol. Sci., 2007, 8, 526–532 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |