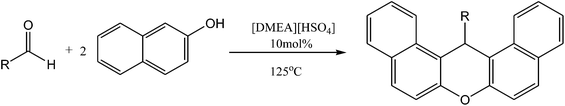
An efficient and reusable ionic liquid catalyst for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes under solvent-free conditions†
Anlian Zhu,
Shukun Bai,
Wei Jin,
Ruixia Liu,
Lingjun Li,
Yang Zhao and
Jianji Wang*
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, P. R. China. E-mail: jwang@henannu.edu.cn; Fax: +86-0373-3329030; Tel: +86-0373-3325805
First published on 4th August 2014
Abstract
In this work, it was found that acidic protic ionic liquid N,N-dimethylaminoethanol hydrosulfate ([DMEA][HSO4]) was an efficient and reusable catalyst for the one-pot synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes under solvent-free conditions. This ionic liquid is very cheap, air and water stable, and can be easily recovered and reused for at least six cycles. The catalytic system described here is a green protocol since in the one-pot synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes, the yield of the target compounds is excellent, operation and work-up procedures are simple, and no volatile organic solvents have been used. It is suggested that the synergetic effect of the cation and the anion of this ionic liquid is the main reason for the high catalytic activity and high chemo-selectivity. Scale-up experiments also suggest that this catalytic system has potential in industrial application.
Introduction
The derivatives of benzoxanthenes have received great attention due to their antibacterial,1 anti-inflammatory2 and antiviral properties,3 and have been used in a wide range of applications in areas including photodynamic therapy for the treatment of tumor cells,4 laser technology,5 visualization of biomolecules,6 and others.7 Recently, various methodologies have been developed for the synthesis of benzoxanthenes, and the most frequently used procedure is through the reaction of 2-naphthol with various aldehydes in the presence of catalyst such as molecular iodine,8 amberlyst-15,9 wet cyanuric chloride,10 tungstophosphoric acid–zirconia composites,11 WCl6,12 SO3H-functionalized acidic ionic liquid,13 silica sulfuric acid,14 sulfonated polyethylene glycol15 and among others.16–20 Although this one-pot procedure makes various benzoxanthenes available, almost all of these catalytic systems suffered from one or more disadvantages such as low yield, long reaction time, tedious work-up procedure, use of special apparatus, excess reagents and expensive/un-reusable catalysts. Thus, the development of efficient, economical and environmentally benign methods for the preparation of benzoxanthenes is still in a great necessity.It has been recognized that choline-based ionic liquids (ILs) are much more biocompatible than their traditional imidazolium-based companions, and they also benefit from wide resources and low preparation cost.21–24 Therefore, these ILs have received increasing attention in organic catalysis and synthesis in recent years.25–30 As our continuous work on the synthesis and utilization of choline-based ionic liquids in sustainable catalysis, the catalytic performances of a series of choline-based ionic liquids on the synthesis of benzoxanthenes were investigated in the present work. For the sake of easy understanding, the structures for the cations of typical choline-based ionic liquids are shown in Scheme 1. It was shown that the catalytic activity and chemical selectivity of the reaction between 2-naphthol and aldehydes were significantly influenced by the combination of cation and anion of the ionic liquids. The ionic liquid with acidic anion of hydrogen sulfate ([HSO4]) and protic cation of N,N-dimethylaminoethanol ([DMEA]) was found to be an efficient and reusable catalyst for the one-pot preparation of a series of 14-aryl-14H-dibenzo[a,j]xanthenes under solvent-free conditions. The reaction mechanism was suggested based on the successful detection of an intermediate and it was shown that cation and anion of the ionic liquid have synergetic effect on its catalytic performance.
Results and discussion
In order to study the catalytic activity of choline-based ionic liquids with different combination of cation and anion on the condensation reactions, the reaction between p-nitrobenzaldehyde and 2-naphthol was initially selected as a model reaction, and the catalytic results were collected in Table 1. It was shown that when the ionic liquids with neutral anions, such as [choline][Cl] and [C4-choline][Cl] (entries 1 and 2, Table 1), were used to promote this reaction at 100 °C, no products can be detected after 10 hours. When the ionic liquids with basic anions, such as [choline][Ac] and [DMEA][Ac] (entries 3 and 4, Table 1), were used as catalyst, the model reaction can proceed fluently at room temperature. After 10 hours, p-nitrobenzaldehyde could be completely used up as monitored by TLC, but 2-naphthol was still in the reaction mixtures. Then, the mixtures were recrystallized using 95% ethanol and a pure product (pale brown color) with the melting point of 210 °C was obtained. 1H NMR detection suggested that only one 2-naphthol was reacted with p-nitrobenzaldehyde and 4a (Scheme 2) was the sole product. It was noted that as the reaction temperature was increased up to 125 °C, another product was produced with melting point of 310 °C (entry 5, Table 1). 1H NMR detection indicated that this is the target compound 3a (Scheme 2), but its final isolated yield was only 70%. Interestingly, as ionic liquid with acidic anion such as [DMEA][HSO4] or [choline][HSO4] (entries 6 and 7, Table 1) was used as catalyst, the product was a mixture of 3a and 4a after 20 min reaction at 125 °C. Nevertheless, as the reaction time was extended to 1 hour, 4a disappeared and turned to the target compound 3a. It was clearly indicated that catalytic activity and chemical selectivity of [DMEA][HSO4] for the reaction were higher than those of [choline][HSO4] due to the stronger acidity of the former30 compared with that of the latter. The above information suggests that in this multi-steps reactions, the first step is both acid and basic favorable, but the subsequent step is acid favorable.| Entry | ILa | Reaction temperature (oC) | Reaction time | Product (yield) |
|---|---|---|---|---|
| a The molar ratio of ionic liquid to substrates was kept at 0.1. | ||||
| 1 | [Choline][Cl] | rt/125 | 10 h | — |
| 2 | [C4-choline][Cl] | rt/125 | 10 h | — |
| 3 | [Choline][Ac] | rt | 10 h | 4a (90%) |
| 4 | [DMEA][Ac] | rt | 10 h | 4a (92%) |
| 5 | [DMEA][Ac] | 125 | 2 h | 3a (70%) |
| 6 | [Choline][HSO4] | 125 | 20 min, 1 h | 3a + 4a (15% + 45%), 3a (85%) |
| 7 | [DMEA][HSO4] | 125 | 20 min, 1 h | 3a + 4a (30% + 50%), 3a (99%) |
The influence of the amount of [DMEA][HSO4] catalyst on the reaction was also studied using the model reaction, and the results were included in Table 2. It can be seen that with increasing molar ratio of [DMEA][HSO4] to substrates from 0.02 to 0.1, the yield for the target compound increased significantly, but further increase of the molar ratio did not have influence on the yield. Therefore, the molar ratio of the ionic liquid to substrates was kept at 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the further experiments.
1 for the further experiments.
Using [DMEA][HSO4] as catalyst, the reactions of 2-naphthol with different aldehydes were used to investigate the scope of the substrates, and the results were given in Table 3. It can be seen that the benzaldehydes and aromatic aldehydes with electron-withdrawing or weak electron-donating substituents such as –NO2, –CH3, –OH and halogens could react smoothly to afford the corresponding products with good to excellent isolated yields (entries 1–8), while the benzaldehydes with strong electron-donating groups such as –OCH3 showed low reactivity and could only get moderate isolated yields with the prolonged reaction time (entries 9 and 10). However, it is interesting to note that when 1,4-phthalaldehyde was used as the substrate, only one aldehyde group was reacted with 2-naphthol, and another aldehyde group was kept intact (entry 11).
| Entry | R | Time | Product | Isolated yield (%) | Mp (°C) | Lit. mp (°C) |
|---|---|---|---|---|---|---|
| a The sixth run of the recovered ionic liquid. | ||||||
| 1 | 4-NO2C6H4 | 1 h | 3a | 99 | 310–312 | 311–312 (ref. 13) |
| 2 | C6H5 | 1 h | 3b | 90 | 178 | 181 (ref. 20) |
| 3 | 3-NO2C6H4 | 1 h | 3c | 98 | 233–234 | 235–237 (ref. 20) |
| 4 | 4-OHC6H4 | 4 h | 3d | 74 | 140–142 | 140 (ref. 14) |
| 5 | 4-CH3C6H4 | 1 h | 3e | 90 | 228 | 227–228 (ref. 13) |
| 6 | 4-ClC6H4 | 1 h | 3f | 92 | 300–301 | 300–302 (ref. 20) |
| 7 | 4-BrC6H4 | 1 h | 3g | 95 | 298 | 295–297 (ref. 12) |
| 8 | 4-FC6H4 | 5 min | 3h | 90 | 250–252 | 238–240 (ref. 13), 265–267 (ref. 20) |
| 9 | 4-CH3OC6H4 | 3 h | 3i | 65 | 206 | 203–205 (ref. 12) |
| 10 | 4-(OH)C6H3-3-(OCH3) | 4 h | 3j | 45 | 198–201 | — |
| 11 | 4-CHOC6H4 | 1 h | 3k | 91 | 328 | — |
The scale up effect of this catalytic system has also been investigated using the model reaction. The results showed that when the reaction scale was increased to 10 mmol, the target compound can still give almost quantitative isolated yields in 1 hour. When the reaction was completed, the target compound can be precipitated and the ionic liquid can be recovered by addition of 1 ml of water and then filtrated. It was found that the amount of IL leaching into the product was negligible. The IL could be recovered after drying of its aqueous solution, and then directly used for the next run. No significant activity decrease was observed after six runs (Table 4), which suggests that this ionic liquid catalyst is recyclable and reusable.
| Runs | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Yield (%) | 99 | 98 | 97 | 96 | 98 | 97 |
According to the above discussion, the cation and the anion of this ionic liquid have synergetic effect on its catalytic performance, and a plausible reaction mechanism is proposed as illustrated in Scheme 3. The findings that the first step is both acid and basic favorable but the subsequent steps are acid favorable may provide some important information for the further catalyst design of the reactions mentioned.
 | ||
| Scheme 3 Proposed reaction mechanism for [DMEA][HSO4] catalyzed synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes. | ||
Conclusions
In this work, we found that ionic liquid [DMEA][HSO4] is an efficient and environmentally friendly catalyst for the one-pot synthesis of a series of 14-aryl-14H-dibenzo[a,j]xanthenes under solvent-free conditions. This catalytic system benefits from easy operation and work-up procedure, wide substrates tolerance and high chemical selectivity. In addition, the catalyst is cheap, feasible, and can be easily recovered and reused for at least six runs without significant activity decrease. It is suggested that the synergetic effect of the cation and anion of the ionic liquid plays a predominant role in the catalytic activity and chemo-selectivity. This finding would be constructive for the rational design of novel catalysts for this type of reactions.Experimental
Synthesis and characterization of the ionic liquids
N,N′-Dimethylaminoethanol (DMEA) and 1-chlorobutane were obtained from Beijing Chem. Reagent Co. and distilled at reduced pressure before use. Choline chloride was also obtained from Beijing Chem. Reagent Co. Sulfuric acid (H2SO4) and acetic acid were purchased from Shanghai Reagent Co., and strong basic ion exchange resin (Ambersep 900 OH) was purchased from Alfa Aesar. 2-Naphthol, benzaldehyde and substituted benzaldehydes were purchased from Aladdin Reagent Co. All of the commercially available reagents were AR grade and used as received unless indicated otherwise. The ionic liquids [DMEA][HSO4] and N,N′-dimethylaminoethanol acetate ([DMEA][Ac]) were synthesized by simple neutralization of N,N′-dimethylaminoethanol with corresponding acids such as sulfuric acid or acetate acid in an equal molar ratio. Choline hydrogen sulfate ([choline][HSO4]) and choline acetate ([choline][Ac]) were prepared by neutralization of corresponding acid with choline hydroxide which was obtained by the ion-exchange of choline chloride using strong basic anion exchange resin. N,N-Dimethyl-N-butylaminoethanol chloride ([C4-choline][Cl]) was prepared by quaternary amination of N,N′-dimethylaminoethanol with chlorobutane. 1H-NMR spectra were recorded on a BRUKER AV-400 instrument at room temperature using TMS as internal standard. Melting points were determined by a XRC-1 Microscopic Melting Point Measurer (Sichuan University Instrument Factory) without correction.Typical procedures for the preparation of 14H-dibenzo[a,j]xanthene derivatives
To a mixture of benzaldehyde (0.5 mmol) and 2-naphthol (1 mmol), a certain amount of choline-based ionic liquid was added as catalyst, and the reaction was monitored by TLC method. After the reaction was completed, the mixtures were cooled to room temperature and washed with water to get the crude product. The pure product can be obtained from the recrystallization using 95% ethanol, and the collected aqueous solution was concentrated by evaporation to regenerate the ionic liquid.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 21373079, 21172058).References
- H. Wang, L. Lu, S. Y. Zhu, Y. Li and W. Cai, Curr. Microbiol., 2006, 52, 1–5 CrossRef CAS PubMed.
- K. Chibale, M. Visser, D. V. Schalkwyk, P. J. Smith, A. Saravanamuthu and A. H. Fairlamb, Tetrahedron, 2003, 59, 2289–2296 CrossRef CAS.
- J. M. Jamison, K. Krabill, A. Hatwalkar, E. Jamison and C. Tsai, Cell Biol. Int. Rep., 1990, 14, 1075–1084 CrossRef CAS.
- R. M. Ion, A. Planner, K. Wiktorowicz and D. Frackowiak, Acta Biochim. Pol., 1998, 45, 833–845 CAS.
- E. Klimtchuk, M. A. J. Rodgers and D. C. Neckers, J. Phys. Chem., 1992, 96, 9817–9820 CrossRef CAS.
- C. G. Knight and T. Stephens, Biochem. J., 1989, 258, 683–687 CAS.
- A. Kumar, S. Sharma, R. A. Maurya and J. Sarkar, J. Comb. Chem., 2010, 12, 20–24 CrossRef CAS PubMed.
- (a) B. Das, B. Ravikanth, R. Ramu, K. Laxminarayana and B. V. Rao, J. Mol. Catal. A: Chem., 2006, 255, 74–77 CrossRef CAS PubMed; (b) M. A. Pasha and P. J. Vaderapura, Bioorg. Med. Chem. Lett., 2007, 17, 621–623 CrossRef CAS PubMed.
- S. Ko and C. F. Yao, Tetrahedron Lett., 2006, 47, 8827–8829 CrossRef CAS PubMed.
- M. A. Bigdeli, M. M. Heravi and G. H. Mahdavinia, Catal. Commun., 2007, 8, 1595–1598 CrossRef CAS PubMed.
- T. S. Rivera, A. Sosa, G. P. Romanelli, M. N. Blanco and L. R. Pizzio, Appl. Catal., A, 2012, 443, 207–213 CrossRef PubMed.
- M. A. Zolfigol, A. R. Moosavi-Zare, P. Arghavani-Hadi, A. Zare, V. Khakyzadeh and G. Darvishi, RSC Adv., 2012, 2, 3618–3620 RSC.
- K. Gong, D. Fang, H. L. Wang, X. L. Zhou and Z. L. Liu, Dyes Pigm., 2009, 80, 30–33 CrossRef CAS PubMed.
- H. R. Shaterian, M. Ghashang and A. Hassankhani, Dyes Pigm., 2008, 76, 564–568 CrossRef CAS PubMed.
- D. Prasad and M. Nath, Catal. Sci. Technol., 2012, 2, 93–96 CAS.
- A. R. Khosropour, M. M. Khodaei and H. Moghannian, Synlett, 2005, 955–958 CrossRef CAS PubMed.
- J. Li, W. Tang, L. Lu and W. Su, Tetrahedron Lett., 2008, 49, 7117–7120 CrossRef CAS PubMed.
- F. Shirini and N. G. Khaligh, Dyes Pigm., 2012, 95, 789–794 CrossRef CAS PubMed.
- N. G. Khaligh, Catal. Sci. Technol., 2012, 2, 2211–2215 Search PubMed.
- S. Kantevari, M. V. Chary, A. P. R. Das, S. V. N. Vuppalapati and N. Lingaiah, Catal. Commun., 2008, 9, 1575–1578 CrossRef CAS PubMed.
- M. Avalos, R. Babiano, P. Cintas, J. L. Jimenez and J. C. Palacios, Angew. Chem., Int. Ed., 2006, 45, 3904–3908 CrossRef CAS PubMed.
- P. Nockemann, B. Thijs, K. Driesen, C. R. Janssen, H. K. L. Van Meervelt, S. B. Kirchner and K. Binnemans, J. Phys. Chem. B, 2007, 111, 5254–5263 CrossRef CAS PubMed.
- J. Pernak, A. Syguda, I. Mirska, A. Pernak, J. Nawrot, A. Pradzynska, S. T. Griffin and R. D. Rogers, Chem.–Eur. J., 2007, 13, 6817–6827 CrossRef CAS PubMed.
- M. Francisco, A. van den Bruinhorst and M. C. Kroon, Angew. Chem., Int. Ed., 2013, 52, 3074–3085 CrossRef CAS PubMed.
- A. L. Zhu, T. Jiang, B. X. Han, J. C. Zhang, Y. Xie and X. M. Ma, Green Chem., 2007, 9, 169–172 RSC.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2006, 8, 784–786 RSC.
- S. Sunitha, S. Kanjilal, P. S. Reddy and R. B. N. Prasad, Tetrahedron Lett., 2007, 48, 6962–6965 CrossRef CAS PubMed.
- A. L. Zhu, L. J. Li, J. J. Wang and K. L. Zhuo, Green Chem., 2011, 13, 1244–1250 RSC.
- A. L. Zhu, R. X. Liu, L. J. Li, L. Y. Li, L. Wang and J. J. Wang, Catal. Today, 2013, 200, 17–23 CrossRef CAS PubMed.
- A. L. Zhu, Q. Q. Li, L. J. Li and J. J. Wang, Catal. Lett., 2013, 143, 463–468 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06521b |
| This journal is © The Royal Society of Chemistry 2014 |