Processing and characterization of ZnO nanowire-grown PBO fibers with simultaneously enhanced interfacial and atomic oxygen resistance properties†
Lei Chena,
Li Liua,
Yunzhe Dua,
Weilu Chenga,
Zhen Hua,
Guangshun Wua,
Qingbo Zhanga,
Chunhua Zhanga and
Yudong Huang*ab
aSchool of Chemical Engineering and Technology, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: ydhuang.hit1@aliyun.com
bState Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150001, P. R. China
First published on 6th November 2014
Abstract
The surface of poly(p-phenylene benzobisoxazole) (PBO) fibers was modified by Zinc oxide nanowires (ZnO NWs) using a mild hydrothermal method to enhance the interfacial properties of PBO fiber/epoxy composites. A functionalization technique was developed to improve the bonding between the PBO fiber and ZnO NWs and was validated by X-ray photoelectron spectroscopy (XPS). Energy dispersive spectrometry (EDS), X-ray diffractometry (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), atomic force microscopy (AFM), wettability testing and single fiber tensile testing were performed to characterize the hybrid fibers. The quantitative relationships between the process parameters (solution concentration ratio and growth time), structure and interfacial shear strength (IFSS) of ZnO NW-grown PBO fibers were systematically investigated. Moreover, the possible interfacial property enhancing reasons were explored. Experimental testing showed that the ZnO NWs interphase developed here offered a significant increase in the IFSS (increased by 50.7%) without degrading the base fiber. It was also shown for the first time that dense ZnO NWs could serve as barriers to protect the PBO fiber underneath from atomic oxygen (AO) erosion, which resulted in their potential applications.
Introduction
Over the past few decades, the field of high performance fibers has witnessed considerable growth. Poly(p-phenylene benzobisoxazole) (PBO) fibers, representative of high-performance fibers, are characterized by high strength, high modulus and high thermal stability.1–8 Based on the superior natures, PBO fibers have attracted significant interest as ideal reinforcements for advanced materials. However, due to the surface smoothness and chemical inertness, PBO fibers have poor interfacial adhesion and low compatibility with resin matrix. Therefore, the surface modification has been a topic of great interest.It is widely accepted that the interfacial region between the reinforcing fiber and resin matrix plays a significant role in determining load transfer mechanisms and ultimately mechanical performance of advanced fiber-reinforced composites.9–13 As such, various approaches have been reported for modifying the interface of fiber composites. Compared to chemical oxidation, plasma treatment and high-energy irradiation, whiskerization is an effective method by growing high strength single crystals such as silicon carbide (SiC) and carbon nanotubes (CNTs) directly onto fibers to increase fiber surface area, create mechanical interlocking, or locally stiffen at the interfacial region.14–18 The most common technique of whiskerization involves coating fibers with CNTs through chemical vapor deposition (CVD) process carried out at a high temperature (>700 °C), which is less likely to integrate with organic fibers.19–21 Electrophoresis deposition (EPD) is another efficient technique for the growth of CNTs on the surface of conductive fibers, whereas it is not applicable to insulation substrates such as aramid and PBO fibers.22,23
Zinc oxide nanowires (ZnO NWs) have attracted considerable attention owing to their unique electrical, optical, magnetic properties and biocompatibility.24–28 Recently, a novel whiskerization method was employed by Lin et al. to grow ZnO NWs on the carbon fiber surface in aqueous solutions at a low temperature (<90 °C).29 They found that the ZnO NWs interphase offered a more than 100% increase in the interfacial strength without degradation of the tensile strength. The report suggested that the bonding between the ZnO NWs and carboxyl functional groups on the carbon fiber surface formed a strong interaction, which laid a firm foundation on enhancing the interfacial strength. Ehlert et al. used a similar method to grow ZnO NWs on the aramid fiber surface.30 Considering the poor adhesion between fibers and ZnO NWs, they developed a functionalization procedure to generate carboxyl functional groups on the fiber surface. It was demonstrated that, following the functionalization, ZnO NWs led to a significant increase in interfacial strength. Different form traditional CVD or EPD of CNTs, ZnO NWs as promising reinforcements, can flexibly grow on various fibers, which extremely extends the field of their applications.31–34
Both the reports mentioned above indicate that the carboxyl functional groups on the fiber surface are critical for the interfacial properties of resulting composites. In the present work, a chemical functionalization method for PBO fibers was developed in this paper to convert hydroxyl to carboxyl functional groups, and then ZnO NWs were directly grown on the PBO fiber surface for the first time. By using the novel method, we explored the possibility to prepare multifunctional PBO fibers with simultaneously enhanced interfacial and atomic oxygen (AO) resistant properties. Seed-to-growth solutions concentrations ratio ([S]/[G]) and growth time were chosen as process parameters to control the surface morphology of ZnO NWs. The correlation between the process parameters, structure and resulting interfacial properties of the hierarchical reinforcements was investigated, and their interfacial property enhancing mechanism was explored. Furthermore, the AO erosion resistance of PBO fibers before and after ZnO NWs growth was studied.
Experimental section
Materials
The PBO fibers (HM) with a single filament diameter of 12 μm were supplied by Toyobo Ltd., Japan. Prior to use, surface sizing and contaminants of PBO fibers were removed by Soxhlet extraction with acetone at 70 °C for 48 h. Zinc acetate dihydrate [Zn(CH3COO)2·2H2O], zinc nitrate hexahydrate [Zn(NO)3·6H2O], sodium hydroxide (NaOH), hexamethylenetetramine (HMTA), sulfuric acid (H2SO4), hydrochloric acid (HCl), chloroacetic acid and ethanol were purchased from Sinopharm Chemical Reagent Co., Ltd., China.Fiber functionalization processes
The PBO fibers were functionalized through three separate surface treatments, which were borrowed from graphene oxide.35 First, the fibers were oxidized in 60 wt% H2SO4 for 3 h to generate active functional groups. After being washed with deionized water for several times, the fibers were submersed in a solution containing 12 g of NaOH and 100 mL of H2O and treated by ultrasonic for 20 min. Then 10 g of chloroacetic acid was added in the solution followed by ultrasonic for 3 h. Finally, an ion-exchange process was performed on the fibers by adding 38 wt% HCl in the solution until pH was 6. The mixture was treated by ultrasonic for another 1 h. Then the resulting fibers (PBO-COOH) were washed with deionized water for several times and dried under vacuum.ZnO NWs growth processes
To synthesize ZnO colloid suspension as quantum dot seeds, a 20 mM solution of NaOH in ethanol and a 12.5 mM solution of Zn(CH3COO)2·2H2O in ethanol were made by vigorously stirring at 50 °C for 10 min separately. Upon cooling, 40 mL of sodium hydroxide solution was then added into 320 mL of ethanol, and 40 mL of zinc acetate dehydrate solution was added into 100 mL of ethanol. Then the two solutions were preheated separately to 65 °C and mixed for 30 min under vigorously stirring. The functionalized PBO fibers were dipped into the seed solution for 15 min, and then annealed at 150 °C for 10 min for a total of three times.The ZnO NWs growth processes ([S]/[G] = 2) were as follows, 3.125 mmol of Zn(NO)3·6H2O and 3.125 mmol of HMTA were dissolved in 500 mL of deionized water, and the solution was heated with vigorous stirring until the temperature of water bath reached 90 °C. The PBO fibers coated with ZnO seeds were immersed into the growth solution for 2, 4, 6 and 8 h, respectively. The resulting fibers were washed with deionized water for several times and dried under vacuum. Similarly, the ZnO NWs growth process was prepared when [S]/[G] ratio was 0.5 and 3.5 to synthesize ZnO NWs of varying morphologies. For convenience, the ZnO NWs-grown PBO fibers were designated as follows, for example, PBO0.5–4 indicated that the [S]/[G] ratio was 0.5 and the growth time was 4 h.
Measurements
The crystal structure of PBO fibers was examined by X-ray diffractometer (XRD, RIGAKU D/MAX-rβ, Japan) with Cu Kα radiation (λ = 1.5406 nm) generated at 40 kV and 100 mA. The elemental chemical states of PBO fibers before and after functionalization process were verified by X-ray photoelectron spectroscopy (XPS, Thermofisher Scienticfic, USA) using a mono-chromated Al Kα source and a pass energy of 50 eV at a base pressure of 2 × 10−9 mbar. The adhesive durability of the ZnO NWs was evaluated by ultrasonic exposure tests, which were carried out in water at 30 °C for 10 min with an ultrasonator (SB3200, Branson).The surface morphology of PBO fibers was observed by scanning electron microscopy (SEM, Hitachi S-4700, Japan) equipped with energy dispersive spectrometer (EDS), which was used for analysis of elemental composition of the ZnO NWs-grown PBO fiber. The surface roughness (Ra) of PBO fibers was measured in an area of 4 mm × 4 mm by atomic force microscopy (AFM, Solver-P47H, NT-MDT, Russia). The electronic analytical balance ALC-110.4 (Shanghai Cany Precision Instrument Co., Ltd.) was used to characterize the wettability of PBO fibers with epoxy resin. The wettability testing lasted 30 min after the prepared samples were hanged over the framework in balance. At first the value was recorded with intervals of 1 s, then 3 s, at last 30 s.
Single fiber pull-out testing was carried out to evaluate IFSS between the PBO fibers and epoxy resin (E-51) matrix by pulling out a fiber from the cured resin droplet. It was performed on an interfacial strength testing instrument (Tohei Sanyon Co., Ltd, Japan) at a crosshead displacement rate of 0.06 mm min−1. The values of IFSS were calculated according to eqn (1):
 | (1) |
Single fiber tensile testing was performed on an electronic mechanical universal material testing machine (Instron 5500R, USA) according to the ASTM D3379-75. At least 60 specimens were measured for each sample, and then the results were analyzed with Weibull statistical method.
AO exposure testing was conducted in a ground-based AO effect simulation facility designed by Beijing University of Aeronautics and Astronautics,36 which employed filament charge and boundary of magnetic field (IFM). During the experiment, all samples were placed on a circular holder in the vacuum chamber of the facility, in which the vacuum pressure was 1.5 × 10−1 Pa. The flux of AO was determined to be 2.1 × 1015 atoms per cm2 s, and the test periods were within 8 h.
Results and discussion
The elemental chemical states of the functionalization processes (untreated PBO, acid-treated PBO and PBO-COOH) are assessed by XPS. The C1s peak positions derive from peak deconvolution results, as shown in Fig. 1a–c. For the untreated PBO fiber (Fig. 1a), we can find that the C1s peaks can be fitted to four peaks with binding energies assigned at 284.5, 285.5, 286.5 and 288.1 eV, which are attributed to C–C, C–N, C–O and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, respectively. For the acid-treated PBO fiber, the XPS results show a new binding energy peak at 289.2 eV, which is attributed to O
C–O, respectively. For the acid-treated PBO fiber, the XPS results show a new binding energy peak at 289.2 eV, which is attributed to O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O. After further functionalization treatment, the peak intensity of O
C–O. After further functionalization treatment, the peak intensity of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O remarkably increases. We activate the acid-treated PBO fiber with chloroacetic acid under strongly basic conditions in order to convert hydroxyl to carboxyl functional groups. In order to analyze the changes of O
C–O remarkably increases. We activate the acid-treated PBO fiber with chloroacetic acid under strongly basic conditions in order to convert hydroxyl to carboxyl functional groups. In order to analyze the changes of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O during the functionalization processes, the concentrations of correlative functional groups, which can be calculated from the related peak areas in XPS C1s spectra, are presented in Table 1. Compared to acid-treated PBO fiber, the concentration of O
C–O during the functionalization processes, the concentrations of correlative functional groups, which can be calculated from the related peak areas in XPS C1s spectra, are presented in Table 1. Compared to acid-treated PBO fiber, the concentration of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O for PBO-COOH increases from 3.4% to 8.73%, which indicates a successful functionalization procedure.
C–O for PBO-COOH increases from 3.4% to 8.73%, which indicates a successful functionalization procedure.
 | ||
| Fig. 1 (a–c) XPS spectra of C1s peaks and (d–f) SEM images of (a), (d) untreated PBO fiber; (b), (e) acid-treated PBO fiber and (c), (f) PBO-COOH. | ||
| Samples | The percentage contents of correlative functional groups (%) | ||||
|---|---|---|---|---|---|
| C–C | C–N | C–O | N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O C–O |
O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O C–O |
|
| Untreated PBO | 66.92 | 15.58 | 11.01 | 6.49 | — |
| Acid-treated | 64.12 | 12.94 | 14.79 | 4.75 | 3.4 |
| PBO-COOH | 62.14 | 13.55 | 10.54 | 5.04 | 8.73 |
Fig. 1d–f show SEM images of untreated, acid-treated and functionalized PBO fibers. It is obvious that the surface of untreated PBO fiber is neat and smooth (Fig. 1d). Fig. 1e and f show the surface morphology of acid-treated PBO fiber and PBO-COOH where some narrow grooves distribute along the axial direction of the fibers. It seems that the functionalization processes do not give rise to any obvious change on PBO fiber in comparison with acid-treated one. Fig. 1f suggests that the structural PBO fibers in this experiment do not suffer etching by the reaction.
To assess the influence of functionalization processes on the adhesive durability of ZnO NWs, ultrasonic exposure tests are performed in this section. As shown in Fig. 2a, the untreated PBO fiber exhibits large adhesive failure of ZnO NWs due to the lack of active functional groups. For the PBO-COOH (Fig. 2b), several micro-cracks on the fiber surface are observed (marked by arrow), while distinct additional peeling is not found. The above results imply that ZnO NWs with high adhesive durability have grown on the fiber surface after the functionalization processes. To assess the influence of functionalization processes on the mechanical properties of PBO fibers, tensile strength (TS) and interfacial shear strength (IFSS) are examined as well. As shown in Fig. 2c, the untreated PBO fiber has a TS of 5.8 GPa and IFSS of 40.4 MPa. After acid treatment, the TS has a small decrease to 5.51 GPa due to the acid etching, and the IFSS has an increase to 48 MPa. After functionalization treatment, the TS of PBO-COOH has no discernable decrease in comparison with acid-treated one. Meanwhile, the IFSS has a slight increase to 52.2 MPa. This is likely due to the fact that abundant carboxyl functional groups on the surface of PBO-COOH provide more chances to react with the epoxy components during the curing process.37,38 Moreover, the mechanical properties of the three samples after ZnO NWs growth are evaluated. It can be seen that the TS of the fibers is not negatively influenced by the NWs growth process. So far, no other whiskerization technique is compatible with organic fibers because of the high-temperature processing required.30,34 In our work, satisfactory in-plane properties of PBO fiber are maintained owing to the advantages of the method. Additionally, it is worth noting that the IFSS of all the samples grown with ZnO NWs is higher than that of another three ones. The improvements could be attributed to two aspects: (1) the enhancement of mechanical interlocking caused by the penetration of stiff ZnO NWs into the resin matrix; (2) the increased bond area with resin matrix caused by the high surface area of ZnO NWs. At the same time, we find that the IFSS for the fibers significantly increases from 44.6 MPa for untreated PBO fiber with ZnO NWs to 60.9 MPa for PBO-COOH with ZnO NWs. This demonstrates that the addition of carboxyl functional groups is critical for forming the strong adhesion between PBO fiber and ZnO NWs, which directly affect the interfacial properties of PBO fiber composites. This result agrees well with the previous report by Sodano.30
 | ||
| Fig. 2 SEM images of ZnO NWs grown on (a) untreated PBO fiber and (b) PBO-COOH after ultrasonic exposure; (c) effect of functionalization processes on the mechanical properties of PBO fibers. | ||
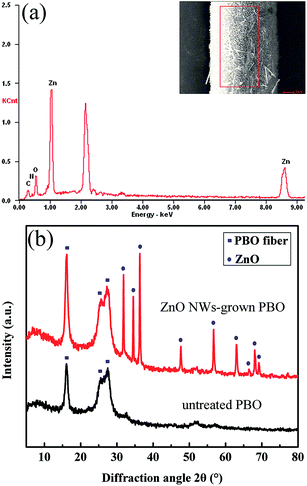
The EDS analysis of a representative ZnO NWs-grown PBO fiber is shown in Fig. 3a. The result reveals that besides carbon, oxygen and nitrogen atoms, zinc atom as the main component appears for it, indicating a successful growth of ZnO NWs on the fiber surface via the mild hydrothermal method.
The XRD patterns of untreated and ZnO NWs-grown PBO fibers are shown in Fig. 3b. The characteristic peaks of PBO fiber appear at 16.1°(200), 25.6°(010) and 27.3°(210) for both samples. For ZnO NWs-grown PBO fiber, characteristic peaks at 31.8°(100), 34.5°(002), 36.3°(101), 47.6°(102), 56.7°(110), 62.9°(103), 66.4°(200), 68.1°(112) and 69.3°(201) can be indexed as the hexagonal wurtzite ZnO phase, which are consistent with the values in the standard card (JCPDS 36-1451). The symmetrical narrow full width at half-maximum (fwhm) of the ZnO peaks indicates that ZnO NWs are well crystallized.39,40
Fig. S1† shows the SEM image of ZnO NWs-grown PBO fiber without cleaning. It can be seen that the ZnO NWs-grown PBO fiber is covered by lots of petaloid ZnO NWs after hydrothermal growth, which have poor adhesion with fiber. They act as “lubricating particles” in the interfacial region of composites, and will lead to the decrease of interfacial properties. Therefore, all of the samples need to be washed by deionized water for several times to remove the unoriented ZnO NWs. Fig. 4 shows SEM images of ZnO NWs-grown PBO fibers of varying morphology. Fig. 4a, c and f show the morphology of ZnO NWs-grown PBO fibers for different [S]/[G] ratios. The density of ZnO NWs is decreased with the increase of [S]/[G] ratio, which is calculated as 100 ± 3, 85 ± 6 and 65 ± 5 ZnO NWs per μm2 for [S]/[G] = 0.5, 2 and 3.5 respectively by counting five different 1 μm2 areas. When [S]/[G] ratio is 3.5, there are many gaps (marked by arrow) among ZnO NWs appear on the fiber surface. Meanwhile, it seems that there are no obvious differences in diameter and length among the three samples, and the detailed dimension results are shown in Fig. S2 in the ESI.† Although low [S]/[G] ratio ([S]/[G] = 0.5) is beneficial to the density of ZnO NWs, it facilitates the nucleation of undesired micron sized rods, which negatively affect the uniformity and quality of ZnO NWs (marked by frame).41 Thus, an appropriate [S]/[G] ratio is important for the morphology of ZnO NWs, as well as the interfacial properties of resulting PBO fiber composites. Fig. 4b–e show the morphology of ZnO NWs-grown PBO fibers for different growth time, and striking differences in the diameter and length of them can be clearly observed. We are able to obtain needle-like structure by keeping the growth time at 4 h or lower. When growth time is 2 h (Fig. 4b), there are numerous of “needle-like” ZnO NWs with short length distribute on the PBO fiber. With an increase of growth time from 2 h to 4 h (Fig. 4c), the length of ZnO NWs becomes longer. We are able to obtain ZnO NWs with significantly increased diameter by increasing growth time to 6 h or longer, as shown in Fig. 4d and e. It can be seen that “rod-like” structure with less aspect ratio is achieved. The detailed results are shown in Fig. S3 in the ESI,† which further indicates that growth time is the main contributor controlling the dimensions of ZnO NWs. As discussed above, the morphology (diameter, length and density) of ZnO NWs can be well controlled by [S]/[G] ratio and growth time.
 | ||
| Fig. 4 SEM images of ZnO NWs-grown PBO fibers: (a) PBO0.5–4; (b) PBO2–2; (c) PBO2–4; (d) PBO2–6; (e) PBO2–8; (f) PBO3.5–4. | ||
Fig. 5 shows the representative cross-sectional SEM and TEM images of ZnO NWs-grown PBO fibers, respectively. As shown in Fig. 5a, each NW has a uniform diameter along its entire length which enables us to calculate its dimension. In fact, one of the challenges in the synthesis of ZnO NWs on flexible fibers is the difficulty in achieving homogeneity, which greatly affects the interfacial properties of PBO fiber composites. Therefore, we have developed a method by which a bundle of PBO fibers are wound around a glass frame, which leads to a good dispersion of individual fibers in the growth solution. From Fig. 5b, we can trace the ZnO NWs bridging PBO fiber and resin matrix. It suggests that the resin matrix can effectively wet the NWs, resulting in a strong mechanical interlocking.29
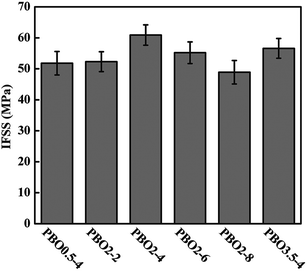
Fig. 6 shows the IFSS of PBO fiber composites with ZnO NWs as a function of [S]/[G] ratio and growth time, respectively. The maximum IFSS for PBO2–4 reaches 60.9 MPa with an increase of 50.7% compared to that of untreated PBO fiber. The IFSS of PBO0.5–4 is 51.8 MPa, and that of PBO3.5–4 is 56.6 MPa, which reveals that both the uniformity and density of ZnO NWs affect the interfacial properties of PBO fiber composites.42 For PBO0.5–4 ([S]/[G] = 0.5), ununiform distribution of ZnO NWs in the interfacial region would cause stress concentration and form defect area, and accordingly weakened the IFSS. For PBO3.5–4 ([S]/[G] = 3.5), low density of ZnO NWs may result in relatively weak mechanical interlocking with resin matrix.43,44 With the increase of growth time, the IFSS of ZnO NWs-grown fiber composites increases firstly with variation from 52.3 to 60.9 MPa, and then decreases to 48.9 MPa, which is highly dependent on the dimension of ZnO NWs. It is interestingly found that the IFSS of PBO fibers with needle-like ZnO NWs (PBO2–2 and PBO2–4) are higher than that of the fibers with rod-like ones (PBO2–6 and PBO2–8). In other words, a nano-scaled interphase consisting of numerous ZnO NWs, with a length below 1 μm and diameter below 100 nm, proves favorable for the improvement of the interfacial properties of PBO fiber composites.
It is worthwhile to mention that many approaches have been employed to enhance the interfacial adhesion between PBO fibers and resin matrix. For example, Wu et al. modified the surface of PBO fibers by oxygen plasma treatment. They found that the IFSS had an increase of 29% when treatment time was 5 min.45 Song et al. reported over 50% improvement of IFSS when vapor acrylic acid was grafted onto the surface of oxygen plasma pretreated PBO fibers.46 Gu et al. proposed a “three-step approach” of methanesulfonic acid/γ-aminopropyl triethoxy silane/glycidylethyl polyhedral oligomeric silsesquioxane (MSA/KH550/POSS) to functionalize the surface of PBO fibers, and found that the IFSS was increased by 26.6% in comparison with that of untreated PBO fiber composites.47 Compared to the previous reports, whiskerization in this work also shows promising results for interface improvement.
In order to further identify the reason for the enhancement of interfacial properties of ZnO NWs-grown PBO fiber composites, the surface roughness and wettability of PBO fibers are examined by AFM and dynamic wetting tests, respectively. AFM results of untreated and ZnO NWs-grown PBO fibers with Ra are presented in Fig. 7a and b, respectively. Compared to untreated PBO fiber, the surface of ZnO NWs-grown one becomes rougher, and Ra increases from 26.6 nm to 63.5 nm accordingly. The ZnO NWs act as anchors to locally stiffen at the interfacial region, which leads to a strong mechanical interlocking between fiber and resin matrix.48,49 Dynamic wetting curves of the two samples in epoxy resin are presented in Fig. 7c. The figure shows that the adsorption weight and wetting velocity of ZnO NWs-grown PBO fiber increase remarkably, indicating an increasing bond area. Compared to previous reports,29,30 these results present concrete evidence that the increased mechanical interlocking and bond area are the main contributors for such an enhancement.
Fig. 7d and e depict the surface morphology of PBO fibers de-bonding from the resin matrix. For the untreated PBO fiber (Fig. 7d), the de-bonded fiber surface is almost clean, on which there are only several epoxy fragments. This means the interface is easily de-bonded due to the weak van der Waals force between fiber and resin matrix. However, in the case of ZnO NWs-grown PBO fiber (Fig. 7e), there are lots of epoxy fragments and micro-cracks are observed. Moreover, it is worthy that some ZnO NWs attached with epoxy fragments can be traced on the fiber surface, and some of them are embedded in the resin matrix (marked by frame), indicating a stronger mechanical interlocking between fiber and resin matrix. Without doubt, the growth of ZnO NWs can form two interfaces one between the PBO fiber and ZnO and a second between the ZnO and resin matrix, and we can imply from the figure that the failure at the ZnO/PBO fiber interface is the major mode,29 which is analogous to the CNTs fracture during carbon fiber de-bonding observed by Hung.50 The possible de-bonding mechanism of PBO fibers is schematically summarized in Fig. 7f. The increased IFSS results suggest that both the interfaces are stronger than the one between PBO fiber and resin matrix of a typical composite.
As mentioned earlier PBO fibers are candidate materials for space applications (reinforcements, tether materials, etc.) used in low earth orbit (LEO), where they will be exposed to damaging AO. Herein, we for the first time exploit the potential of ZnO NWs in improving the resistance of PBO fibers to AO erosion. Fig. 8a–d show the surface morphology of untreated and ZnO NWs-grown PBO fibers exposed to AO for the same time, respectively. The untreated PBO fiber is severely eroded and roughened, giving a “corduroy-like” appearance (Fig. 8a and b). In contrast, the surface of ZnO NWs-grown PBO fiber (PBO2–4) is not heavily eroded (Fig. 8c and d). We can observe from Fig. 8e that the TS of ZnO NWs-grown PBO fiber decreases slower than that of untreated one when they are exposed to AO. After 8 h AO accelerated aging, the TS retention ratio of untreated and ZnO NWs-grown PBO fibers is 68.4% and 77.5%, respectively. Obviously, the TS retention of ZnO NWs-grown PBO fiber is higher than that of untreated one. Dense ZnO NWs could act as high energy barriers to protect the PBO fiber underneath from AO migration and diffusion.
 | ||
| Fig. 8 SEM images of (a–b) untreated PBO fiber and (c–d) ZnO NWs-grown PBO fiber exposed to AO for 8 h; (e) TS retention of PBO fibers after AO accelerated aging. | ||
Conclusions
In summary, we have successfully grown ZnO NWs on PBO fibers by varying the seed-to-growth solutions concentrations ratio ([S]/[G]) and growth time through a hydrothermal method at a relatively low temperature. To address the weak adhesion between the fiber and ZnO NWs, a functionalization technique was developed to produce carboxyl functional groups on the fiber surface. The correlation between the process parameters, structure and resulting interfacial properties of the hierarchical reinforcements was systematically investigated. Experiment results suggested that the IFSS was greatly improved with increasing amplitude of 50.7% after the growth of ZnO NWs. The increased bonding area and mechanical interlocking were the main contributors for such an enhancement. It was also demonstrated that the low temperature required for the growth of ZnO NWs did not degrade the TS of base fiber. In addition, for the first time the potential of ZnO NWs to improve the AO erosion resistance of PBO fibers in LEO was explored, which of ZnO NWs-grown PBO fiber was superior to that of untreated one. We anticipate that this study will pave a novel way for preparing multifunctional PBO fibers with simultaneously enhanced interfacial and AO resistant properties.Acknowledgements
The authors acknowledge the financial support by the National High Technology Research and Development Program of China (863 Program, no. 2012AA03A212), the Chang Jiang Scholars Program and the National Science Foundation of China (no. 51073047, no. 91016015, no. 51103031, no. 51173032, no. 51273050).References
- J. F. Wolf and F. E. Arnold, Macromolecules, 1981, 14, 909–915 CrossRef.
- S. G. Wierschke, J. R. Shoemaker, P. D. Haaland, R. Pachter and W. W. Adams, Polymer, 1992, 33, 3357–3368 CrossRef CAS.
- Y. Takahashi, Macromolecules, 1999, 32, 4010–4014 CrossRef CAS.
- D. W. Tomlin, A. V. Fratini, M. Hunsaker and W. W. Adams, Polymer, 2000, 41, 9003–9010 CrossRef CAS.
- S. F. Ran, C. Burger, D. F. Fang, X. H. Zong, S. Cruz, B. Chu, B. S. Hsiao, R. A. Bubeck, K. Yabuki, Y. Teramoto, D. C. Martin, M. A. Johnson and P. M. Cunniff, Macromolecules, 2002, 35, 433–439 CrossRef CAS.
- J. M. Park, D. S. Kim and S. R. Kim, J. Colloid Interface Sci., 2003, 264, 431–445 CrossRef CAS.
- F. Guo, Z. Z. Zhang, H. J. Zhang and W. M. Liu, Composites, Part A, 2009, 40, 1305–1310 CrossRef PubMed.
- Z. Hu, J. Li, P. Y. Tang, D. L. Li, Y. J. Song, Y. W. Li, L. Zhao, C. Y. Li and Y. D. Huang, J. Mater. Chem., 2012, 22, 19863–19871 RSC.
- V. Carlier, M. Sclavons, A. M. Jonas, R. Jérôme and R. Legras, Macromolecules, 2001, 34, 3725–3729 CrossRef CAS.
- S. L. Gao, E. Mäder and S. F. Zhandarov, Carbon, 2004, 42, 515–529 CrossRef CAS PubMed.
- L. M. Liao, X. Wang, P. F. Fang, K. M. Liew and C. X. Pan, ACS Appl. Mater. Interfaces, 2011, 3, 534–538 CAS.
- J. C. Fan, Z. X. Shi, L. Zhang, J. L. Wang and J. Yin, Nanoscale, 2012, 4, 7046–7055 RSC.
- W. H. Liao, H. W. Tien, S. T. Hsiao, S. M. Li, Y. S. Wang, Y. L. Huang, S. Y. Yang, C. C. M. Ma and Y. F. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 3975–3982 CAS.
- G. M. Wu and Y. T. Shyng, Composites, Part A, 2004, 35, 1291–1300 CrossRef PubMed.
- C. H. Zhang, Y. D. Huang and Y. D. Zhao, Mater. Chem. Phys., 2005, 92, 245–250 CrossRef CAS PubMed.
- K. Tamargo-Martínez, A. Martínez-Alonso, M. A. Montes-Morán and J. M. D. Tascó, Compos. Sci. Technol., 2011, 71, 784–790 CrossRef PubMed.
- D. Liu, P. Chen, M. Chen and Z. Liu, Surf. Coat. Technol., 2012, 206, 3534–3541 CrossRef CAS PubMed.
- L. Chen, Z. Hu, L. Liu and Y. D. Huang, RSC Adv., 2013, 3, 24664–24670 RSC.
- E. T. Thostenson, W. Z. Li, D. Z. Wang, Z. F. Ren and T. W. Chou, J. Appl. Phys., 2002, 91, 6034–6037 CrossRef CAS PubMed.
- Z. G. Zhao, L. J. Ci, H. M. Cheng and J. B. Bai, Carbon, 2005, 43, 663–665 CrossRef CAS PubMed.
- P. Lv, Y. Y. Feng, P. Zhang, H. M. Chen, N. Q. Zhao and W. Feng, Carbon, 2011, 49, 4665–4673 CrossRef CAS PubMed.
- E. Bekyarova, E. T. Thostenson, A. Yu, H. Kim, J. Gao, J. Tang, H. T. Hahn, T. W. Chou, M. E. Itkis and R. C. Haddon, Langmuir, 2007, 23, 3970–3974 CrossRef CAS PubMed.
- S. Y. Wang and R. A. W. Dryfe, J. Mater. Chem. A, 2013, 1, 5279–5283 CAS.
- S. Xu and Z. L. Wang, Nano Res., 2011, 4, 1013–1098 CrossRef CAS.
- T. Belagodu, E. A. Azhar and H. B. Yu, Nanoscale, 2012, 4, 7330–7333 RSC.
- S. M. Niu, Y. F. Hu, X. N. Wen, Y. S. Zhou, F. Zhang, L. Lin, S. H. Wang and Z. L. Wang, Adv. Mater., 2013, 25, 3701–3706 CrossRef CAS PubMed.
- C. X. Guo, Y. Q. Dong, H. B. Yang and C. M. Li, Adv. Energy Mater., 2013, 3, 997–1003 CrossRef CAS.
- J. R. Lee, S. Y. Choi, S. J. Bae, S. M. Yoon, J. S. Choi and M. J. Yoon, Nanoscale, 2013, 5, 10275–10282 RSC.
- Y. R. Lin, G. Ehlert and H. A. Sodano, Adv. Funct. Mater., 2009, 19, 2654–2660 CrossRef CAS.
- G. J. Ehlert and H. A. Sodano, ACS Appl. Mater. Interfaces, 2009, 1, 1827–1833 CAS.
- C. H. Xue, R. L. Wang, J. Zhang, S. T. Jia and L. Q. Tian, Mater. Lett., 2010, 64, 327–330 CrossRef CAS PubMed.
- J. H. Bae, M. K. Song, Y. J. Park, J. M. Kim, M. L. Liu and Z. L. Wang, Angew. Chem., Int. Ed., 2011, 50, 1683–1687 CrossRef CAS PubMed.
- U. Galan, Y. R. Lin, G. J. Ehlert and H. A. Sodano, Compos. Sci. Technol., 2011, 71, 946–954 CrossRef CAS PubMed.
- G. J. Ehlert, U. Galan and H. A. Sodano, ACS Appl. Mater. Interfaces, 2013, 5, 635–645 CAS.
- X. M. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. J. Dai, Nano Res., 2008, 1, 203–212 CrossRef CAS PubMed.
- X. H. Zhao, Z. G. Shen, Y. S. Xing and S. L. Ma, Polym. Degrad. Stab., 2005, 88, 275–285 CrossRef CAS PubMed.
- M. Delamar, G. Désarmot, O. Fagebaume, R. Hitmi, J. Pinson and J. M. Savéant, Carbon, 1997, 35, 801–807 CrossRef CAS.
- J. Zhu, J. D. kim, H. Q. Peng, J. L. Margrave, V. N. Khabashesku and E. V. Barrera, Nano Lett., 2003, 3, 1107–1113 CrossRef CAS.
- T. J. Athauda and R. R. Ozer, Cryst. Growth Des., 2013, 13, 2680–2686 CAS.
- T. J. Athauda, P. Hari and R. R. Ozer, ACS Appl. Mater. Interfaces, 2013, 5, 6237–6246 CAS.
- K. Kong, B. K. Deka, S. K. Kwak, A. Oh, H. Kim, Y. B. Park and H. W. Park, Composites, Part A, 2013, 55, 152–160 CrossRef CAS PubMed.
- X. Q. Zhang, X. Y. Fan, C. Yan, H. Z. Li, Y. D. Zhu, X. T. Li and L. P. Yu, ACS Appl. Mater. Interfaces, 2012, 4, 1543–1552 CAS.
- Q. Y. Peng, Y. B. Li, X. D. He, H. Z. Lv, P. A. Hu, Y. Y. Shang, C. Wang, R. G. Wang, T. Sritharan and S. Y. Du, Compos. Sci. Technol., 2013, 74, 37–42 CrossRef CAS PubMed.
- Q. Y. Peng, Y. B. Li, Y. J. Ding, Q. Y. Peng, C. Wang, R. G. Wang, T. Sritharan, X. D. He and S. Y. Du, Compos. Sci. Technol., 2013, 85, 36–42 CrossRef PubMed.
- G. M. Wu, Mater. Chem. Phys., 2004, 85, 81–87 CrossRef CAS PubMed.
- B. Song, L. H. Meng and Y. D. Huang, Appl. Surf. Sci., 2012, 258, 5505–5510 CrossRef CAS PubMed.
- J. W. Gu, T. Bai, J. Dang, J. J. Feng and Q. Y. Zhang, Polym. Compos., 2014, 35, 611–616 CrossRef CAS.
- F. Zhao and Y. D. Huang, J. Mater. Chem., 2011, 21, 2867–2870 RSC.
- F. Zhao, Y. D. Huang, L. Liu, Y. P. Bai and L. W. Xu, Carbon, 2011, 49, 2624–2632 CrossRef CAS PubMed.
- K. H. Hung, W. S. Kuo, T. H. Ko, S. S. Tzeng and C. F. Yan, Composites, Part A, 2009, 40, 1299–1304 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06468b |
| This journal is © The Royal Society of Chemistry 2014 |