Structural, electrical and optical properties of Mg-doped CuAlO2 films by pulsed laser deposition
Abstract
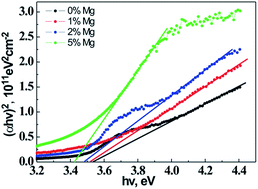
CuAl1−xMgxO2 (x = 0, 0.01, 0.02, 0.05) films were deposited on sapphire and fused silica substrates by pulsed laser deposition and underwent annealing in an Ar atmosphere at the temperature of 1000 °C. The effects of Mg concentration on the structural, morphological, electrical and optical properties were investigated by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), UV-visible-NIR spectrophotometry and a Hall effect measurement system. The results indicate that Mg is successfully doped into the CuAlO2 film with p-type conduction after annealing. Single and pure-phase CuAl1−xMgxO2 (x = 0, 0.01, 0.02, 0.05) films with c-axis orientation are obtained on sapphire substrates in the studied doping range, and a secondary phase is not detected by XRD measurements. The substitution of Mg2+ ions in Al3+ sites induces lattice distortion and results in the decline of the crystalline quality. The Hall effect measurement reveals that the carrier concentration increases and Hall mobility decreases with the increase of Mg doping concentration. The resistivity of the CuAl1−xMgxO2 films decreases and then increases with increasing Mg doping concentration from 0 to 5%. The minimum resistivity of 11.45 Ω cm is obtained at room temperature for the CuAl1−xMgxO2 films with x = 2%. The optical transmittance gradually reduces with the increase of Mg concentration due to the enhancement of absorption of incident photons. The optical band gaps of CuAl1−xMgxO2 films are found to decrease from 3.54 to 3.43 eV as the Mg doping concentration increased from 0 to 5% due to the formation of impurity energy levels.

 Please wait while we load your content...
Please wait while we load your content...