 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Immobilization of helicene onto carbon substrates through electropolymerization of [7]helicenyl-thiophene†
J.
Hrbac
ab,
J.
Storch
*c,
V.
Halouzka
b,
V.
Cirkva
c,
P.
Matejka
d and
J.
Vacek
*b
aDepartment of Physical Chemistry, Palacky University, tr. Svobody 26, 77146 Olomouc, Czech Republic
bDepartment of Medical Chemistry and Biochemistry, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic. E-mail: jan.vacek@upol.cz
cInstitute of Chemical Process Fundamentals of the AS CR, v.v.i., Rozvojova 135, 165 02 Prague 6, Czech Republic. E-mail: storchj@icpf.cas.cz
dDepartment of Physical Chemistry, Institute of Chemical Technology, Prague, Technicka 5, 166 28 Prague 6, Czech Republic
First published on 16th September 2014
Abstract
In this work, monomeric 3-([7]helicen-9-yl)thiophene was synthesized and used for the preparation of a polymeric film by electrosynthesis. The polymer prepared was characterized using Fe(CN)63−/4− and Ru(NH3)63+/2+ redox probes, scanning electron microscopy and vibrational spectroscopy. The electropolymerization approach presented here provides a new option for the immobilization of helicenes onto solid supports.
Helicenes are molecules with a broad range of applications in physical and chemical research and technology development.1 One of the main fields of interest is the design of helicene derivatives and polymers, resulting in novel species with tailored physico-chemical properties leading to better stability, solubility, adsorbability and higher applicability in analytical chemistry, materials science and optoelectronics.2 The newly prepared helicene derivatives were shown to be effective in the development of materials with chiroptical properties useful for molecular-based electronic applications. Other studies were focused on the interaction of helicenes with biomacromolecules and the preparation of helicene foldamers. With respect to the unique optical properties of helicenes, selected derivatives also have potential as fluorescence active probes and dyes.1
The main goal of this work was the preparation of a monomeric helicene conjugate with thiophene that could be useful for the preparation of polymer systems utilizing an electrosynthetic approach. The main focus of this work is the thiophene-mediated electropolymerization of the monomer and thus the formation of a thin film of helicene on solid supports. Thiophenes or their polymeric structures are intensively studied species with applications in electronics, the development of optical and electrochemical sensors, bioanalysis and biomedicine in general.3
To the best of our knowledge, there has been no study dealing with a simple polymer backbone e.g. poly(thiophene), poly(phenylene-vinylene) etc., bearing helicene moieties as a conductive or optically active component. The incorporation of optically active helicenes into a semiconducting polymeric backbone could lead to the discovery of as yet unexplored new materials combining chirality with electrical conductivity, e.g. circularly polarized devices.4
The first step was the preparation of a conjugate of thiophene and racemic [7]helicene. The main prerequisite was the fact that thiophene undergoes electrooxidation, resulting in the formation of poly(thiophene) conductive films onto solid supports (electrodes).5 The monomeric unit, 3-([7]helicen-9-yl)thiophene, was synthesized using the methodology developed by ourselves6 starting from commercially available 9-bromo[7]helicene by means of Suzuki coupling with thienylboronic acid, catalyzed by XPhos and Pd2(dba)3 with K3PO4 as a base, for details see Section 1 in ESI.†
In the next phase of the experimental work, 3-([7]helicen-9-yl)thiophene was used for the electrosynthesis of the polymer (Fig. 1) onto carbon materials. Glassy carbon (GC) and carbon fiber (CF) electrodes were used. The electropolymerization process was performed by immersing the above electrodes into an acetonitrile/0.1 M tetrabutylammonium perchlorate (ACN/TBAP) electrolyte containing 1 × 10−3 M 3-([7]helicen-9-yl)thiophene monomer. The electrode was then polarized from 0 to +2.5 V (vs. Ag/AgCl3 M KCl) using cyclic voltammetry (10 cycles) at a scan rate of 100 mV s−1 (for further details, see Section 2 in ESI†). The acquired cyclic voltammetry (CV) records at the GC electrode are shown in Fig. 2. The formation of the polymer layer is indicated by a decrease in the anodic currents at a potential of around +1.5 V in the 2nd and consecutive CV scans. Poly[3-([7]helicen-9-yl)thiophene] is formed (Fig. 2, dotted line) more easily than poly(thiophene) (Fig. 2, full line) under the same experimental conditions. The thiophene CV records are in good agreement with previously published data under similar experimental (nonaqueous) conditions.7 The [7]helicene moiety could also undergo an electrooxidation reaction at carbon surfaces, but without forming a deposit, as shown using the 9-bromo[7]helicene control sample (see Section 3 in ESI†). In addition to the GC electrode, the same procedure was used with a CF electrode, because CF can be easily examined microscopically for visualizing the poly[3-([7]helicen-9-yl)thiophene] deposition onto the carbon material. The formation of the deposit was observed by scanning electron microscopy (SEM). Untreated and electrosynthetically treated CFs are marked A/A′ and B/B′ in Fig. 3, respectively (for other details, see Section 3 in ESI†). The thicknesses of electrodeposited layers were measured under different scan rates and number of CV scans (Fig. S5 in ESI†). It was shown that there is a positive correlation between polymer formation and decreasing scan rate and increasing number of scans applied. After the application of 10 scans at a scan rate of 5 mV s−1 the thickness of the deposit formed onto CFs was ca. 4.5 μm.
 | ||
| Fig. 2 Background corrected cyclic voltammograms of 1 × 10−3 M 3-([7]helicen-9-yl)thiophene (dotted line) and thiophene (full line) at GC electrode in ACN/0.1 M TBAP, 10 cycles, scan rate 100 mV s−1. | ||
 | ||
| Fig. 3 Scanning electron micrographs of CFs. (A/A′) untreated and (B/B′) coated with poly[3-([7]helicen-9-yl)thiophene]. For other micrographs and control experiments, see Section 3 in ESI.† | ||
Both electrodes were modified with poly[3-([7]helicen-9-yl)thiophene] repeatedly, where Al2O3 (0.05 μm) mechanical polishing was used for GC electrode surface renewal between individual experiments. Regeneration of the surfaces of fragile CFs was performed according to a recently published protocol based on sinusoidal-wave potential cycling.8
Under the same polymerization conditions as in Fig. 2, the interfacial properties of the poly[3-([7]helicen-9-yl)thiophene] layer were examined using a ferricyanide Fe(CN)63−/4− redox probe (Fig. 4). CV measurement was performed with 1 mM potassium ferricyanide at a bare and a polymer-modified electrode. The bare GC electrode gives a typical redox couple, which is in agreement with previously published data.9 After electrode modification, the complete disappearance of the ferricyanide redox couple current response indicates that the deposit formed onto the GC electrode is compact and defect-free, i.e. impermeable to the low-molecular redox probe. The ferricyanide redox probe exchanges electrons via the ‘surface-sensitive’ mechanism.10 In addition to ferricyanide, a hexaammineruthenium(III) chloride Ru(NH3)63+/2+ probe,11 the electrochemistry of which is not ‘surface-sensitive’,10 was used to evaluate the electron transfer properties of the electrodeposited layer. The current response of Ru(NH3)63+/2+ was not completely reduced after GC surface modification by the polymer. This finding probably indicates that the poly[3-([7]helicen-9-yl)thiophene] deposit is partially able to transfer electrons (‘electron tunneling’ at a limited rate), which is in agreement with the conductive properties of poly(thiophene) films and strictly π-conjugated helicene systems.12 A detailed investigation of the selectivity of poly[3-([7]helicen-9-yl)thiophene] to the redox probes compared to poly(thiophene) is shown in Section 2 in ESI.†
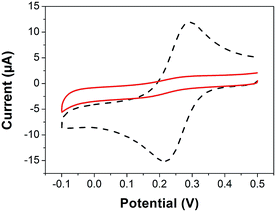 | ||
| Fig. 4 CV records of 1 mM potassium ferricyanide recorded in 0.1 M KCl on poly[3-([7]helicen-9-yl)thiophene] modified (full line) and bare (dashed line) GC electrode. The poly[3-([7]helicen-9-yl)thiophene] layer was prepared under the conditions given in Fig. 2. For other details and control experiments, see Section 2 in ESI.† | ||
For characterization of the polymer in comparison with the 3-([7]helicen-9-yl)thiophene monomer, FT Raman and FT-IR spectra were recorded. The polymer solid was prepared using an Au strip via the same procedure as in Fig. 2. During electrolysis at the Au strip, the polymer was released as a precipitate that was centrifuged, washed, dried and used for spectral experiments. The Raman spectrum of the precipitated product exhibits broadened characteristics, corresponding to a markedly disordered polymer material. However, the features of the helicene skeleton are usually observed at shifted positions, becoming comparable with nanocrystalline carbon or graphene-based materials (e.g. the bands at 1603, 1362, and 1327 cm−1)13 (Fig. 5). The Raman characteristics of the polythiophene moiety are weak, but the positions are comparable with the data published for substituted poly(thiophene)s, e.g. (ref. 14). Similar observations were obtained in the infrared spectra, for details see Section 4 in ESI.†
 | ||
| Fig. 5 Comparison of Raman spectra of polymer with 3-([7]helicen-9-yl)thiophene monomer (offset scale). | ||
Conclusions
Here we synthesized the conjugate of [7]helicene with thiophene where thiophene functionality enables a single-step electropolymerization procedure and thus the immobilization of [7]helicene onto solid supports. The electrochemically generated poly[3-([7]helicen-9-yl)thiophene] formed a compact deposit on carbon materials under nonaqueous conditions, which was shown using cyclic voltammetry, SEM and vibrational spectroscopy. This polymer opens up new possibilities for the development of novel helicene-modified surfaces with applications in materials science and electronics, e.g. stationary phases for separation approaches, circularly polarized light detection and circularly polarized electroluminescence devices.4 The conditions driving the deposit thickness, role of the helicene moiety in thiophene-mediated electropolymerization reaction, details on the polymer structure and preparation of the thiophene conjugates with optically pure forms of [7]helicene will be investigated in more detail in subsequent studies.Acknowledgements
This work was supported by the Ministry of Industry and Trade of the Czech Republic (FR-TI4/457, J.V.) and by the Technology Agency of the Czech Republic (TA01010646, J.S.). V.H. was supported by the student grant IGA_PrF_2014032 from Palacky University. The authors wish to thank Dr Milan Vujtek, Department of Experimental Physics, Palacky University for assistance with SEM measurements.Notes and references
- (a) R. Amemiya and M. Yamaguchi, Chem. Rec., 2008, 8, 116–127 CrossRef CAS PubMed; (b) M. Gingras, Chem. Soc. Rev., 2013, 42, 968–1006 RSC; (c) M. Gingras, Chem. Soc. Rev., 2013, 42, 1051–1095 RSC; (d) M. Gingras, G. Félix and R. Peresutti, Chem. Soc. Rev., 2013, 42, 1007–1050 RSC; (e) K. Kamikawa, J. Synth. Org. Chem., 2014, 72, 58–67 CrossRef CAS; (f) A. Urbano, Angew. Chem., Int. Ed., 2003, 42, 3986–3989 CrossRef CAS PubMed.
- Y. Shen and C. F. Chen, Chem. Rev., 2013, 112, 1463–1535 CrossRef PubMed.
- (a) A. Berlin, B. Vercelli and G. Zotti, Polym. Rev., 2008, 48, 493–530 CrossRef CAS; (b) R. S. Bobade, J. Polym. Eng., 2011, 31, 209–215 CAS; (c) N. K. Guimard, N. Gomez and C. E. Schmidt, Prog. Polym. Sci., 2007, 32, 876–921 CrossRef CAS PubMed; (d) Y. He, J. Hou, H. Fan and Y. Li, Chem. Bull., 2007, 70, 248–256 CAS; (e) T. Higashihara and M. Ueda, Macromol. Res., 2013, 21, 257–271 CrossRef CAS PubMed; (f) C. Li and G. Shi, ACS Appl. Mater. Interfaces, 2013, 5, 4503–4510 CrossRef CAS PubMed; (g) X. Liu, Q. Fan and W. Huang, Biosens. Bioelectron., 2011, 26, 2154–2164 CrossRef CAS PubMed; (h) P. Sista, K. Ghosh, J. S. Martinez and R. C. Rocha, J. Nanosci. Nanotechnol., 2014, 14, 250–272 CrossRef CAS PubMed; (i) H. J. Wang, C. P. Chen and R. J. Jeng, Materials, 2014, 7, 2411–2439 CrossRef CAS PubMed; (j) C. Zanardi, F. Terzi and R. Seeber, Anal. Bioanal. Chem., 2013, 405, 509–531 CrossRef CAS PubMed.
- (a) Y. Yang, R. C. Da Costa, M. J. Fuchter and A. J. Campbell, Nat. Photonics, 2013, 7, 634–638 CrossRef CAS; (b) Y. Yang, R. C. Da Costa, D. M. Smilgies, A. J. Campbell and M. J. Fuchter, Adv. Mater., 2013, 25, 2624–2628 CrossRef CAS PubMed.
- A. Kausar, S. Zulfiqar and M. I. Sarwar, Polym. Rev., 2014, 54, 185–267 CrossRef CAS.
- J. Zadny, P. Velisek, M. Jakubec, J. Sykora, V. Cirkva and J. Storch, Tetrahedron, 2013, 69, 6213–6218 CrossRef CAS PubMed.
- (a) M. Can, K. Pekmez, N. Pekmez and A. Yildiz, Synth. Met., 1999, 104, 9–17 CrossRef CAS; (b) H. J. Kim, M. H. Piao, S. H. Choi, C. H. Shin and Y. T. Lee, Sensors, 2008, 8, 4110–4118 CrossRef CAS PubMed.
- V. Halouzka, J. Hrbac, D. Jirovsky, D. Riman, P. Jakubec, Z. Bartosova, V. Masek, P. Mojzes and J. Vacek, Curr. Anal. Chem., 2013, 9, 305–311 CAS.
- (a) P. Heiduschka and J. Dittrich, Electrochim. Acta, 1992, 37, 2573–2580 CrossRef CAS; (b) M. M. Radhi, Res. Chem. Intermed., 2014, 40, 1975–1987 CrossRef CAS PubMed; (c) M. M. Radhi, W. T. Tan, M. Z. B. Ab Rahman and A. B. Kassim, J. Chem. Eng. Jpn., 2010, 43, 927–931 CrossRef CAS.
- R. L. McCreery, Chem. Rev., 2008, 108, 2646–2687 CrossRef CAS PubMed.
- M. Zhou, L. P. Guo, Y. Hou and X. J. Peng, Electrochim. Acta, 2008, 53, 4176–4184 CrossRef CAS PubMed.
- (a) J. P. Ferraris and T. R. Hanlon, Polymer, 1989, 30, 1319–1327 CrossRef CAS; (b) L. Pospisil, F. Teply, M. Gal, L. Adriaenssens, M. Horacek and L. Severa, Phys. Chem. Chem. Phys., 2010, 12, 1550–1556 RSC; (c) L. Rulíšek, O. Exner, L. Cwiklik, P. Jungwirth, I. Starý, L. Pospíšil and Z. Havias, J. Phys. Chem. C, 2007, 111, 14948–14955 CrossRef.
- (a) C. Castiglioni, M. Tommasini and G. Zerbi, Philos. Trans. R. Soc., A, 2004, 362, 2425–2459 CrossRef CAS PubMed; (b) I. Childres, L. A. Jauregui, W. Park, H. Cao and Y. P. Chen, Raman Spectroscopy of Graphene and Related Materials, in New Developments in Photon and Materials Research, ISBN: 978-1-62618-384-1, ed. J. I. Jang, Nova Science Pub., 2013 Search PubMed.
- F. Chen, G. Shi, J. Zhang and M. Fu, Thin Solid Films, 2003, 424, 283–290 CrossRef CAS.
Footnote |
| † Electronic supplementary information (SI) available: Section 1: synthesis, NMR and MS data for 3-([7]helicen-9-yl)thiophene, Section 2: details on electrochemical experiments, Section 3: SEM experimental details, Section 4: vibrational spectroscopic characterization. See DOI: 10.1039/c4ra06283c |
| This journal is © The Royal Society of Chemistry 2014 |

