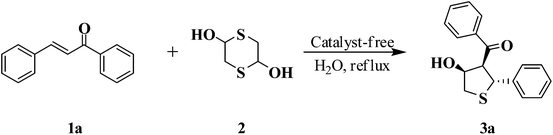
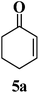
Catalyst-free synthesis of trisubstituted tetrahydrothiophenes in water via a cascade sulfa-Michael/aldol (Henry) type reaction†
Jian Songa,
Jamia Mossb,
Da-Cheng Yanga,
Zhi Guan*a and
Yan-Hong He*a
aSchool of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, P. R. China. E-mail: guanzhi@swu.edu.cn; heyh@swu.edu.cn; Fax: +86-23-68254091
bDepartment of Chemistry, College of Saint Benedict and Saint John's University, MN 56374, USA
First published on 13th October 2014
Abstract
A simple, efficient, eco-friendly and catalyst-free procedure was developed for the construction of trisubstituted tetrahydrothiophenes via sulfa-Michael/aldol (Henry) cascade reaction in water. The protocol, simply utilizing readily-available starting materials under clean reaction conditions, provided an alternative and highly attractive approach to a series of tetrahydrothiophene derivatives from a wide range of substrates in good yields (up to 93%) with excellent diastereoselectivities (up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
1. Introduction
Catalyst-free reactions in aqueous medium have received a great deal of attention because water is considerably safe, readily available, non-toxic, non-flammable, environmentally benign, sustainable and cheap. Apart from the economic and environmental benefits, water also exhibits unique physical and chemical activity that lead to unique selectivity compared with the properties of organic solvents. Thus, the development of organic reactions in water medium is required in the present days.1–3 Moreover, due to the reduction of pollution, low cost and easy isolation, catalyst-free synthesis methods have also gained great interest not only for laboratory synthesis but also in the chemical industry.1,4–7The tetrahydrothiophene moiety is the core structural component of many natural products, bioactive compounds and important synthetic intermediates that form thiophene derivatives through dehydration and aromatization.8,9 Many S-heterocycles are biologically active such as the essential coenzyme biotin,10 the atypical anti-psychotic Olanzapine,11 the anti-HIV nucleoside,12 and the analogues of penicillins13 (Fig. 1). Recently, great efforts have been made to develop methods for the preparation of thiophene derivatives.14,15 1,4-Dithiane-2,5-diol was widely used as an efficient synthon for a straightforward synthesis of functionalized tetrahydrothiophenes via sulfa-Michael/aldol type reactions.2,3,16–21 However, all these methods required catalysts. Besides organocatalytic asymmetric transformations,8,17,18 Et3N16,20,21 and tertiary amine immobilized polyacrylonitrile fiber9,19 were utilized as catalysts. In this context, we report an eco-friendly, synthetically efficient, and catalyst-free protocol for the preparation of tetrahydrothiophene in aqueous medium. Despite the presence of three stereocenters in the product, it is interesting to find the formation of only two diastereomers with one of them formed predominantly, and in most cases, excellent diastereoselectivity (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was obtained.
1) was obtained.
Based on some pioneer work,16–18,20,21 1,4-dithiane-2,5-diol (2), the mercaptoacetaldehyde dimer, was used as a convenient and efficient synthon providing an in situ generated mercaptoacetaldehyde able to add to α,β-unsaturated ketones via a sulfa-Michael addition. The derived ketone adducts provided tetrahydrothiophen scaffolds through a subsequent intramolecular aldol reaction (Scheme 1).
 | ||
| Scheme 1 Cascade sulfa-Michael/aldol reaction for the synthesis of trisubstituted tetrahydrothiophenes. | ||
2. Results and discussion
The reaction of chalcone (1a) and 1,4-dithiane-2,5-diol (2) was chosen as a model reaction. Solvent screening was performed at 25 °C under catalyst-free conditions (Table 1). It could be seen that the model reaction in polar protic solvents such as EtOH, isopropanol and H2O afforded product with good diastereoselectivity, and especially in water the best diastereomeric ratio (dr) of >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was obtained (Table 1, entries 1, 2 and 5). On the contrary, the reaction in aprotic solvents such as MeCN and CH2Cl2 gave product with low diastereoselectivity (Table 1, entries 3 and 4). In general, the reaction in organic solvents provided product in better yields than in water, probably due to the poor solubility of substrates in water at 25 °C. From the perspective of environment-friendliness and in order to get best diastereoselectivity, we chose water as a promising solvent. Next, influence of temperature was investigated (Table 1, entries 5–10). To our surprise, when the reaction was taken place under reflux conditions in water, a good yield of 80% and a perfect dr of >99
1 was obtained (Table 1, entries 1, 2 and 5). On the contrary, the reaction in aprotic solvents such as MeCN and CH2Cl2 gave product with low diastereoselectivity (Table 1, entries 3 and 4). In general, the reaction in organic solvents provided product in better yields than in water, probably due to the poor solubility of substrates in water at 25 °C. From the perspective of environment-friendliness and in order to get best diastereoselectivity, we chose water as a promising solvent. Next, influence of temperature was investigated (Table 1, entries 5–10). To our surprise, when the reaction was taken place under reflux conditions in water, a good yield of 80% and a perfect dr of >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were received. Therefore, reaction in refluxing water was selected as an optimal condition for further study.
1 were received. Therefore, reaction in refluxing water was selected as an optimal condition for further study.
| Entry | Solvent | T (°C) | Yieldb (%) | drc (3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a) 4a) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol) and 2 (0.5 mmol) in solvent (1.0 mL) for 24 h.b Isolated yield.c Determined by 1H NMR analysis. | ||||
| 1 | EtOH | 25 | 50 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | Isopropanol | 25 | 36 | 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | MeCN | 25 | 63 | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | CH2Cl2 | 25 | 46 | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | H2O | 25 | 8 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | H2O | 30 | 10 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | H2O | 40 | 32 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | H2O | 60 | 48 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | H2O | 80 | 72 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | H2O | Reflux | 80 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
In order to further improve the yield of the cascade reaction, effects of molar ratio of substrates on the model reaction were investigated (Table 2). The product was obtained only in 56% yield when the molar ratio of chalcone (1a) to 1,4-dithiane-2,5-diol (2) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 2, entry 1). A good yield of 83% with >99
1 (Table 2, entry 1). A good yield of 83% with >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio was obtained when the molar ratio was increased to 1
1 diastereomeric ratio was obtained when the molar ratio was increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 (1a
1.6 (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Table 2, entry 4). Further increasing the molar ratio of 1a
2) (Table 2, entry 4). Further increasing the molar ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 led to a decrease of the yield (Table 2, entries 5–7). Moreover, using excess amount of chalcone (1a) did not give a better yield (Table 2, entries 8 and 9). It is interesting that the change of the molar ratio of substrates did not influence the diastereoselectivity, and the excellent dr of >99
2 led to a decrease of the yield (Table 2, entries 5–7). Moreover, using excess amount of chalcone (1a) did not give a better yield (Table 2, entries 8 and 9). It is interesting that the change of the molar ratio of substrates did not influence the diastereoselectivity, and the excellent dr of >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was observed in all cases. Thus 1
1 was observed in all cases. Thus 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 was chosen as the optimum molar ratio of chalcone (1a) to 1,4-dithiane-2,5-diol (2).
1.6 was chosen as the optimum molar ratio of chalcone (1a) to 1,4-dithiane-2,5-diol (2).
| Entry | 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Yieldb (%) | drc |
|---|---|---|---|
| a Reaction conditions: 1a and 2 in water (1.0 mL) at reflux temperature for 24 h.b Isolated yield.c Determined by 1H NMR analysis. | |||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
56 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
66 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
79 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
83 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8 |
69 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0 |
65 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.0 3.0 |
33 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
62 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
52 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
To find out a suitable volume of solvent for the reaction, we investigated the effect of the volume of water on the reaction (Table 3). The reaction in 0.5 mL water gave a lower diastereoselectivity of 5.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 3, entry 1), while all the reactions in 1.0–3.0 mL water gave a perfect dr of >99
1 dr (Table 3, entry 1), while all the reactions in 1.0–3.0 mL water gave a perfect dr of >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 3, entries 2–5), further demonstrating that enough water was essential for the reaction to get a good diastereoselectivity. At same time, the reaction in 0.5 mL water gave a low yield probably due to the insufficient solubility of substrates in 0.5 mL water even at reflux temperature (Table 3, entry 1). When the water volume from 1.0 mL to 3.0 mL was used, good yields were obtained with slightly difference (Table 3, entries 2–5). Based on these experiments, we chose 1.5 mL of water as the optimal solvent volume.
1 (Table 3, entries 2–5), further demonstrating that enough water was essential for the reaction to get a good diastereoselectivity. At same time, the reaction in 0.5 mL water gave a low yield probably due to the insufficient solubility of substrates in 0.5 mL water even at reflux temperature (Table 3, entry 1). When the water volume from 1.0 mL to 3.0 mL was used, good yields were obtained with slightly difference (Table 3, entries 2–5). Based on these experiments, we chose 1.5 mL of water as the optimal solvent volume.
The time course of the catalyst-free sulfa-Michael/aldol cascade reaction was investigated under the optimal conditions (Fig. 2). The best yield of 87% was obtained after 12 h, and prolonging the reaction time did not lead to an increase of the yield.
Finally, to explore the scope and limitations of this catalyst-free protocol for the synthesis of trisubstituted tetrahydrothiophenes, we investigated the sulfa-Michael/aldol cascade reactions between various chalcones (1) and 1,4-dithiane-2,5-diol (2) under optimized reaction conditions. As summarized in Table 4, in all cases, the reactions proceeded smoothly affording the corresponding products in generally good yields and high levels of diastereoselectivity. In general, chalcones with electron-withdrawing substituents for R1 gave better yields than those with electron-donating substituents (Table 4, entries 2, 5, 8, 9 and 10). For example, the reaction of 1,4-dithiane-2,5-diol with 4-chlorochalcone gave a yield of 82% after 11 h (Table 4, entry 2). However, the reaction with 4-methoxychalcone provided a lower yield of 60% after 12 h (Table 4, entry 10). On the contrary, the chalcone with an electron-withdrawing substituent –Cl for R2 gave a lower yield after longer reaction time than that with an electron-donating substituent –Me (Table 4, entries 11 and 12). The position of substituents on chalcones had no obvious effect on the yields but great effect on the diastereomeric ratios. Chalcones with R1 as ortho substituents on benzene ring displayed lower diastereoselectivity (Table 4, entries 4, 7, 14 and 15), while all the chalcones with R1 as meta or para substituents on benzene ring showed perfect diastereoselectivity of >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. One of the possible reasons for diastereoselectivity was the steric hindrance of the chalcones with ortho substituents. Disubstituted chalcones were also used in the cascade sulfa-Michael/aldol reaction, giving good yields and good to excellent dr (Table 4, entries 13, 14 and 15).
1 dr. One of the possible reasons for diastereoselectivity was the steric hindrance of the chalcones with ortho substituents. Disubstituted chalcones were also used in the cascade sulfa-Michael/aldol reaction, giving good yields and good to excellent dr (Table 4, entries 13, 14 and 15).
| Entry | R1 | R2 | Product | Time (h) | Yieldb (%) | drc (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.3 mmol) and 2 (0.48 mmol) in water (1.5 mL) at reflux temperature.b Isolated yield.c Determined by 1H-NMR analysis.d Yield of the isolated products (3 + 4). | ||||||
| 1 | H | H | 3a | 12 | 87 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 4-Cl | H | 3b | 11 | 82 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 3-Cl | H | 3c | 11 | 83 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 2-Cl | H | 3d, 4d | 12 | 89d | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 4-Br | H | 3e | 10 | 75 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 3-Br | H | 3f | 10 | 76 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 2-NO2 | H | 3g, 4g | 12 | 74d | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 4-NO2 | H | 3h | 13 | 82 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 4-Me | H | 3i | 12 | 61 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 4-OMe | H | 3j | 12 | 60 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | H | 4-Me | 3k | 12 | 80 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | H | 4-Cl | 3l | 15 | 71 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 4-Cl | 4-Cl | 3m | 18 | 70 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 2,4-DiCl | H | 3n, 4n | 16 | 87d | 5.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | 2,6-DiCl | H | 3o, 4o | 18 | 91d | 7.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
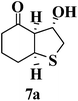
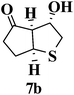
Besides chalcones, some cyclic and acyclic α,β-unsaturated ketones were also used in the cascade sulfa-Michael/aldol reaction process resulting in the efficient preparation of mono-cyclic and bicyclic tetrahydrothiophene derivatives in good yields with high diastereoselectivity. As shown in Table 5, 1,4-dithiane-2,5-diol (2) reacted with cyclohexenone giving 6a as a major product (Table 5, entry 1). Treatment of 2 with cyclopentenone gave rise to a bicyclic derivative 6b as a major product (72% yield for 6b + 7b, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) (Table 5, entry 2). 6a and 6b could be easily separated from their diastereoisomers 7a and 7b by silica gel column chromatography. The single stereoisomers 6c and 6d were obtained in yields of 84% and 53% through reactions of 2 with (S)-carvone 5c and β-ionone 5d, respectively (Table 5, entries 3 and 4).
1 dr) (Table 5, entry 2). 6a and 6b could be easily separated from their diastereoisomers 7a and 7b by silica gel column chromatography. The single stereoisomers 6c and 6d were obtained in yields of 84% and 53% through reactions of 2 with (S)-carvone 5c and β-ionone 5d, respectively (Table 5, entries 3 and 4).
To our surprise, this catalyst-free cascade reaction could also be applied to nitroalkenes. The cascade sulfa-Michael/Henry reactions between 1,4-dithiane-2,5-diol (2) and various trans-β-nitroalkenes (8) afforded the corresponding 3-nitro-2-substituted tetrahydrothiophenes in water at reflux temperature. The reaction between 1,4-dithiane-2,5-diol (2) and phenyl nitroolefin gave the product in a yield of 66% with 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 6, entry 1). Heterocyclic nitroolefins such as thienyl nitroolefin and furyl nitroolefin reacted with 1,4-dithiane-2,5-diol (2) both giving the corresponding products in good yields of 93% but with low diastereoselectivity (Table 6, entries 2 and 3).
1 dr (Table 6, entry 1). Heterocyclic nitroolefins such as thienyl nitroolefin and furyl nitroolefin reacted with 1,4-dithiane-2,5-diol (2) both giving the corresponding products in good yields of 93% but with low diastereoselectivity (Table 6, entries 2 and 3).
The workup processes for the above reactions involved organic solvents. To test the possibility for the purification of the product with water, the model reaction of chalcone (1a) and 1,4-dithiane-2,5-diol (2) was used as an example. After completion of the reaction, the reaction mixture was cooled to room temperature, and a big amount of solid generated. TLC analysis showed that the solid contained the product (3a), 1,4-dithiane-2,5-diol and a very small amount of chalcone. The mother liquid contained most of excess 1,4-dithiane-2,5-diol, but nearly no product was detected in it. Thus, after sucking out the mother liquid by pipet and washing the solid with hot water, the pure product was obtained in a good yield of 89% with >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. This experiment confirmed that tetrahydrothiophene derivatives could be synthesized and purified under totally organic solvent-free conditions. (For the pictures of TLC analysis, and 1H-NMR and HPLC charts of the product purified with water, please see the ESI.†)
1 dr. This experiment confirmed that tetrahydrothiophene derivatives could be synthesized and purified under totally organic solvent-free conditions. (For the pictures of TLC analysis, and 1H-NMR and HPLC charts of the product purified with water, please see the ESI.†)
The product yields and dr values in the present catalyst-free method versus previously reported catalytic alternatives were compared (Tables 7–9). For the reactions between chalcones and 1,4-dithiane-2,5-diol, the only one reference is about asymmetric catalysis using a chiral bifunctional squaramide as a catalyst to synthesize enantioenriched trisubstituted tetrahydrothiophenes.18 For the cascade sulfa-Michael/aldol reactions with other α,β-unsaturated ketones and the cascade sulfa-Michael/Henry reactions, the previously reported catalytic methods are non-enantioselective.9,16,20 From the comparison, it could be seen that the reactions under the present catalyst-free conditions generally obtained similar yields with higher diastereoselectivities than the previously reported reactions catalyzed by chiral bifunctional squaramide, Et3N and tertiary amine immobilized fiber, respectively.
| Entry | Product | Catalyst-free method | Chiral squaramide catalysis (ref. 18) | ||
|---|---|---|---|---|---|
| Yield (%) | dr (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Yield (%) | dr (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
||
| 1 | 3a | 87 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 3b | 82 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 3c | 83 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
79 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 3e | 75 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
76 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 3i | 61 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 3j | 60 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 3l | 71 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Entry | Product | Catalyst-free method | Et3N catalysis (ref. 20) | ||
|---|---|---|---|---|---|
| Yield (%) | dr (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
Yield (%) | dr (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
||
| 1 | 6a, 7a | 67 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 6b, 7b | 72 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 6c | 84 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
45 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 6d | 53 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
45 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Finally, based on the above experimental results and the activation model reported previously,1,18 a plausible reaction mechanism was proposed. As shown in Scheme 2, the water-activated mercaptoacetaldehyde and enone undergo the intermolecular sulfa-Michael addition, and the derived ketone adduct provides tetrahydrothiophen scaffold through a subsequent intramolecular aldol reaction. We assumed that hydrogen bonds between the substrates and water play an important role for the observed diastereoselectivity.
3. Conclusion
We developed a facile, highly atom-efficient, and eco-friendly procedure for the synthesis of tetrahydrothiophene derivatives via cascade sulfa-Michael addition/intramolecular aldol reaction sequence in water under catalyst-free conditions. Trisubstituted tetrahydrothiophenes were obtained in good yields (up to 93%) with excellent diastereoselectivity (up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) from simple and readily available starting materials. The method could also be extended to cascade sulfa-Michael/Henry reactions to afford 3-nitro-2-substituted tetrahydrothiophenes. In this procedure water was not only used as an environmentally-benign solvent, but it also exhibited unique property that leaded to perfect diastereoselectivity in most cases. This protocol provided a clean approach to various tetrahydrothiophene derivatives.
1) from simple and readily available starting materials. The method could also be extended to cascade sulfa-Michael/Henry reactions to afford 3-nitro-2-substituted tetrahydrothiophenes. In this procedure water was not only used as an environmentally-benign solvent, but it also exhibited unique property that leaded to perfect diastereoselectivity in most cases. This protocol provided a clean approach to various tetrahydrothiophene derivatives.
4. Experimental
General procedure for the cascade sulfa-Michael/aldol (Henry) reaction (products 3a–o, 4d, 4g, 4n, 4o, 6a–d, 7a–b, 9a–c and 10a–c)
A 10 mL round-bottomed flask was charged with 1,4-dithiane-2,5-diol 2 (0.48 mmol), chalcone 1 or α,β-unsaturated ketone 5 or nitroalkene 8 (0.3 mmol), and deionized water (1.5 mL). The resultant mixture was stirred at reflux temperature for a specific time. Progress of the reaction was monitored by thin layer chromatography (TLC). The reaction mixture was diluted with CH2Cl2 (10 mL), and the suspended solid was removed by filtration. A solution of saturated ammonium chloride (10 mL) was added to the filtrate and the aqueous layer was extracted with CH2Cl2 (2 × 5 mL). The combined organic layers were washed with water (10 mL) and brine (10 mL) and then dried over anhydrous Na2SO4. Volatiles were removed at reduced pressure, and the resulting residue was purified by silica gel column chromatography with petroleum ether/ethyl acetate as eluent to give the product.The procedure for the cascade sulfa-Michale/aldol reaction (workup with water, product 3a)
A 10 mL round-bottomed flask was charged with 1,4-dithiane-2,5-diol 2 (0.48 mmol), chalcone 1a (0.3 mmol) and deionized water (1.5 mL). The resultant mixture was stirred at reflux temperature for 12 h, and then cooled to room temperature. A big amount of solid generated. The mother liquid was sucked out by pipet, the solid was washed twice by taking in 10 mL of 80 °C water under ultrasound for 5 min, and then the water phase was sucked out by pipet. The solid was dried at 50 °C overnight to give the pure product in a yield of 89% with >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.
1 dr.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21276211).References
- M. B. Gawande, V. D. B. Bonifacio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522–5551 RSC.
- R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302–6337 CrossRef CAS PubMed.
- A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725–748 CrossRef CAS PubMed.
- J. Liu, M. Lei and L. Hu, Green Chem., 2012, 14, 2534–2539 RSC.
- G. Choudhary and R. K. Peddinti, Green Chem., 2011, 13, 276–282 RSC.
- J. Zhang, Z. Cui, F. Wang, Y. Wang, Z. Miao and R. Chen, Green Chem., 2007, 9, 1341–1345 RSC.
- S. Kumar, P. Sharma, K. K. Kapoor and M. S. Hundal, Tetrahedron, 2008, 64, 536–542 CrossRef CAS PubMed.
- J. Tang, D. Q. Xu, A. B. Xia, Y. F. Wang, J. R. Jiang, S. P. Luo and Z. Y. Xu, Adv. Synth. Catal., 2010, 352, 2121–2126 CrossRef CAS.
- C. Xu, J. Du, L. Ma, G. Li, M. Tao and W. Zhang, Tetrahedron, 2013, 69, 4749–4757 CrossRef CAS PubMed.
- P. J. De Clercq, Chem. Rev., 1997, 97, 1755–1792 CrossRef CAS PubMed.
- J. Hartwig, S. Ceylan, L. Kupracz, L. Coutable and A. Kirschning, Angew. Chem., Int. Ed., 2013, 52, 9813–9817 CrossRef CAS PubMed.
- J. Wirsching, J. Voss, G. Adiwidjaja, A. Giesler and J. Kopf, Eur. J. Org. Chem., 2001, 2001, 1077–1087 CrossRef.
- J. W. Johnson, D. P. Evanoff, M. E. Savard, G. Lange, T. R. Ramadhar, A. Assoud, N. J. Taylor and G. I. Dmitrienko, J. Org. Chem., 2008, 73, 6970–6982 CrossRef CAS PubMed.
- K. C. Majumdar and S. Mondal, Heterocycles in Natural Product Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2011, pp. 377–401 Search PubMed.
- J. Wolf and B. Schulze, Advances in Heterocyclic Chemistry, ed. R. K. Alan, Academic Press, 2007, vol. 94, pp. 215–304 Search PubMed.
- C. J. O' Connor, M. D. Roydhouse, A. M. Przybył, M. D. Wall and J. M. Southern, J. Org. Chem., 2010, 75, 2534–2538 CrossRef PubMed.
- Y. Su, J.-B. Ling, S. Zhang and P.-F. Xu, J. Org. Chem., 2013, 78, 11053–11058 CrossRef CAS PubMed.
- J.-B. Ling, Y. Su, H.-L. Zhu, G.-Y. Wang and P.-F. Xu, Org. Lett., 2012, 14, 1090–1093 CrossRef CAS PubMed.
- L. Ma, L. Yuan, C. Xu, G. Li, M. Tao and W. Zhang, Synthesis, 2013, 45, 45–52 CAS.
- N. Baricordi, S. Benetti, V. Bertolasi, C. De Risi, G. P. Pollini, F. Zamberlan and V. Zanirato, Tetrahedron, 2012, 68, 208–213 CrossRef CAS PubMed.
- S. Vivekkumar, P. Prasanna and S. Perumal, Tetrahedron Lett., 2013, 54, 6651–6655 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General methods, the pictures of TLC analysis and charts of 1H-NMR and HPLC for the product 3a purified with water, 1H NMR and 13C NMR for all the products, and HRMS for unknown compounds. See DOI: 10.1039/c4ra06273f |
| This journal is © The Royal Society of Chemistry 2014 |