Iridium(iii) catalyzed trifluoroacetoxylation of aromatic hydrocarbons†
Abstract
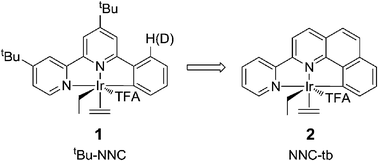
A tridentate, NNC-tb (where NNC-tb = 2-(pyridin-2-yl)benzo[h]quinoline) ligated IrIII complex (NNC-tb)Ir(Ph)(4-MePy)(TFA), 11 along with analogues are very active for CH activation as evidenced by rapid catalytic H/D exchange between benzene and trifluoroacetic acid – d1 (DTFA). The complexes were examined with a variety of oxidants for the catalytic conversion of benzene to phenyltrifluoroacetate. Herein, the synthesis and characterization of (NNC-tb)Ir complexes is described along with the reactivity of these complexes towards arenes and alkanes.
- This article is part of the themed collection: The Carbon–Metal Bond and C–H Metalation

 Please wait while we load your content...
Please wait while we load your content...