Copper-catalyzed acyloxylation of the C(sp3)–H bond adjacent to an oxygen by a cross-dehydrogenative coupling approach†
Abstract
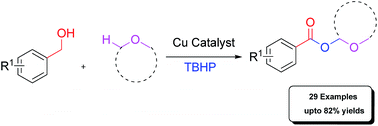
The acyloxylation of the C(sp3)–H bond adjacent to oxygen by adopting a copper catalyzed dehydrogenative cross-coupling reaction between simple ethers and benzyl alcohols has been disclosed. The advantages of the dehydrogenative cross-coupling reaction are the adoption of unactivated ethers as substrates and good tolerance of many functional groups.

 Please wait while we load your content...
Please wait while we load your content...