Synthesis and characterization of self-cross-linkable and bactericidal methacrylate polymers having renewable cardanol moieties for surface coating applications†
Yong-Seok Choi‡
a,
Ki-Hyun Kim‡b,
Dong-Gyun Kima,
Hee Joong Kima,
Sang-Ho Chac and
Jong-Chan Lee*a
aSchool of Chemical and Biological Engineering and Institute of Chemical Processes, Seoul National University, 599 Gwanak-ro, Gwanak-gu, Seoul 151-744, Republic of Korea. E-mail: jongchan@snu.ac.kr
bMaterials R&D Center, Samsung Advanced Institute of Technology, Samsung Electronics Co., Ltd., Nongseo-dong, Giheung-gu, Gyeonggi-do 446-712, Republic of Korea
cDepartment of Chemical Engineering, Kyonggi University, 94-6 Yiui-dong Yeongton-gu, Suwon, Gyeonggi-do 443-760, Republic of Korea
First published on 26th August 2014
Abstract
Polymers containing a renewable cardanol moiety were prepared via radical polymerization of 2-hydroxy-3-cardanylpropyl methacrylate (HCPM) and methyl methacrylate (MMA), where HCPM was synthesized by a reaction of cardanol with glycidyl methacrylate in the presence of a base catalyst. Incorporation of the cardanol moiety into PMMA was found to increase the thermal and mechanical stability of the brittle PMMA. When the cardanol based polymers were irradiated with UV light, the mechanical stability increased further because cross-linked networks were formed between the double bonds in the cardanol moieties. Cross-linked polymer films containing the cardanol moiety exhibited high gloss and transparency to visible light. Cardanol-containing polymers with and without the cross-linked networks and other cardanol-based polymers such as poly(cardanyl acrylate) and poly(2-acetoxy-3-cardanylpropyl methacrylate) all showed high antibacterial activity against Escherichia coli (E. coli), indicating that the disappearance of double bonds and/or the structure changes of connecting groups do not diminish the intrinsic bactericidal properties of the cardanol moieties.
1. Introduction
Recently much effort has been devoted to develop renewable materials to replace petroleum-derived materials because of environmental and economic issues in sustainable development.1–3 In the last decade, several ‘renewable’ polymeric materials based on lactic acid, soybean oil, polysaccharide, and cardanol have been investigated for many potential applications.4–10 Among them, cardanol is one of the important renewable resources, having a C15 unsaturated hydrocarbon chain with one to three double bonds at the meta position of the phenol group. It can be obtained readily by the distillation of cashew nut shell liquid, a by-product of cashew nut production.5,7,11 Cardanol has been used and studied in various applications such as brake linings, coatings, surfactants, varnishes and so on. In addition, it has been also reported that cardanol has the antibacterial property, although the detailed antibacterial mechanism still remains unclear.4,12–14 Although there have been detailed studies and applications for the polymers having other bactericidal groups such as with quaternary ammonium salt and N-halamine, and the polymer nanocomposites containing silver nanoparticles, there has been no systematic studies on antibacterial behaviour of cardanol-based polymers including the thermal, surface, and optical properties.46–49Previously others prepared cardanol-based polymers via the simply mixing cardanol with formaldehyde, enzymatic oxidative polymerization, and the radical polymerization of acrylic monomers having cardanol moieties.4–7,15–17 In this study, we prepared a series of copolymers (PHMs) containing methyl methacrylate (MMA) and 2-hydroxy-3-cardanylpropyl methacrylate (HCPM) moieties. The cardanol-containing monomer (HCPM) was synthesized by the reaction of cardanol and glycidyl methacrylate. MMA was chosen as the co-monomeric unit because poly(methyl methacrylate) (PMMA) has been used widely in coating materials due to its high transparency and impact strength.18 Stable cross-linked PHM films could be prepared using a drop-casting method followed by a UV curing process, and their thermal, surface, optical, and antibacterial properties were investigated. We believe that this is the first report of a systematic study of the bactericidal properties of (meth)acrylic polymers containing cardanol moieties.
2. Experimental
2.1. Materials
Cardanol was provided by Mercury Co., Ltd. (India). Glycidyl methacrylate and triethylamine were purchased from TCI Co., Ltd. (Japan). Methyl methacrylate (MMA), azobisisobutyronitrile (AIBN), acryloyl chloride, and acetyl chloride were purchased from Sigma-Aldrich Co., Ltd. (USA). Potassium hydroxide (KOH) and sodium hydroxide (NaOH), N,N-dimethylacetamide (DMAc) were obtained from Daejung Chemicals & Metals Co., Ltd. (Korea). Tetrahydrofuran (THF) was dried by refluxing over sodium and benzophenone followed by distillation. Toluene was distilled over calcium hydride. Escherichia coli (E. coli; ATCC 8739) was obtained from American Type Culture Collection (ATCC). Bacto™ Agar and Difco™ Nutrient Broth were obtained from Becton, Dickinson and Company (BD). All other reagents were obtained from standard vendors and used as received.2.2. Synthesis of 2-hydroxy-3-cardanylpropyl methacrylate (HCPM)
To a DMAc solution (30 mL) containing cardanol (10 g, 33 mmol) and potassium hydroxide (1.85 g, 33 mmol), glycidyl methacrylate (9.44 g, 66 mmol) was added and reacted under nitrogen (N2) atmosphere for 24 h at room temperature. The reaction was finished by dropping a few drops of a concentrated HCl solution and then DMAc was evaporated in a low-pressure environment. The crude product was dissolved in methylene chloride (MC) and transferred to a separatory funnel. After extraction with 0.5 N HCl solution, the MC layer was dried over anhydrous magnesium sulfate and filtered. The obtained product was purified by silica gel column chromatography (ethyl acetate–n-hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 vol%). The yield was 49% (7.18 g).
6 vol%). The yield was 49% (7.18 g).
1H NMR (300 MHz, CDCl3, trimethylsilane (TMS) ref): δ = 0.88 (t, J = 6.78 Hz, 3H, –CH3), 1.20–1.40 (m, CH3(CH2)12CH2–), 1.60 (m, 2H, CH3(CH2)12CH2CH2–), 1.97 (s, 3H, –OC(O)C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 2.02 (m, –CH2CH2CH2CH
CH2), 2.02 (m, –CH2CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2–), 2.57 (t, J = 8.04 Hz, 2H, –OC6H4CH2–), 2.75–2.90 (m, –CH2CH
CHCH2–), 2.57 (t, J = 8.04 Hz, 2H, –OC6H4CH2–), 2.75–2.90 (m, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH
CHCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.94–4.40 (m, 5H, –OCH2CH(OH)CH2OC(O)–), 4.97–5.80 (m, –CH2CH
CH–), 3.94–4.40 (m, 5H, –OCH2CH(OH)CH2OC(O)–), 4.97–5.80 (m, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2–), 5.62 and 6.26 (s, 2H, –OC(O)C(CH3)
CHCH2–), 5.62 and 6.26 (s, 2H, –OC(O)C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 6.67–6.83 (m, 3H, aromatic), 7.19 (t, J = 7.5 Hz, 1H, aromatic). FT-IR: 3471 cm−1 (O–H stretching vibration), 3010 cm−1 (C–H vibration of the unsaturated hydrocarbon), 1720 cm−1 (C
CH2), 6.67–6.83 (m, 3H, aromatic), 7.19 (t, J = 7.5 Hz, 1H, aromatic). FT-IR: 3471 cm−1 (O–H stretching vibration), 3010 cm−1 (C–H vibration of the unsaturated hydrocarbon), 1720 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration (α,β-unsaturated ester)), 1261 cm−1 (C(Ar)–O–C asymmetric stretching vibration (m-alkyl phenol)), 1049 cm−1 (C(Ar)–O–C symmetric stretching vibration (m-alkyl phenol)), 775 cm−1 (–CH2– rocking vibration), 721 cm−1 (–(CH2)n–, n > 3; rocking vibration), 694 cm−1 (aromatic out of plane C–H deformation vibration of meta-substituted benzene). Mass m/z calculated C28H44O4+: 444.3, found 444.0.
O stretching vibration (α,β-unsaturated ester)), 1261 cm−1 (C(Ar)–O–C asymmetric stretching vibration (m-alkyl phenol)), 1049 cm−1 (C(Ar)–O–C symmetric stretching vibration (m-alkyl phenol)), 775 cm−1 (–CH2– rocking vibration), 721 cm−1 (–(CH2)n–, n > 3; rocking vibration), 694 cm−1 (aromatic out of plane C–H deformation vibration of meta-substituted benzene). Mass m/z calculated C28H44O4+: 444.3, found 444.0.
2.3. Synthesis of copolymers containing HCPM and MMA monomeric units (PHMs)
The copolymers containing HCPM and MMA monomeric units were abbreviated as PHM#, where the # is the molar composition (%) of HCPM in the polymers. The following procedure was used for the preparation of PHM47 containing 47 mol% of HCPM and 53 mol% of MMA monomeric units, respectively. HCPM (3.0 g, 6.75 mmol), MMA (0.675 g, 6.75 mmol), AIBN (0.12 g, 0.70 mmol), and THF (18 mL) were added to a round-bottomed flask equipped with a condenser and a magnetic stirring bar. The flask was purged with N2 and sonicated for 10 min to degas the mixture and remove dissolved oxygen. Then, the mixture was refluxed with stirring. After 24 h of the polymerization, the solution was exposed to air. The crude product was poured into excess of distilled water/methanol (2/1). The dissolution–precipitation procedure was repeated three times, yielding a yellowish wax (2.37 g). The Mn and PDI of PHM47 (gel permeation chromatography (GPC), polystyrene standards, THF as an eluent) were 4900 g mol−1 and 1.50, respectively. Other PHMs with different compositions were prepared using the same procedure except the monomer feed ratios as shown in Table 1.| Samples | Composition (HCPM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MMA) MMA) |
Mnb (× 10−3, RI) | PDIb | ||
|---|---|---|---|---|---|
Feed (%) (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) mol) |
In polymera (%) (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) mol) |
In polymer (%) (wt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt) wt) |
|||
| a Composition of HCPM versus MMA determined by 1H NMR.b Determined by GPC using refractive index (RI) detector and calibrated with linear polystyrene standards (THF). | |||||
| PHM100 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
8.2 | 2.30 |
| PHM47 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 53 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
4.9 | 1.51 |
| PHM10 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
6.2 | 3.13 |
| PMMA | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
7.1 | 1.59 |
1H NMR (300 MHz, CDCl3, TMS ref): δ = 0.8–1.1 (3H, –CH3), 1.20–1.90 (m, CH3(CH2)12CH2– and backbone), 1.55 (m, 2H, CH3(CH2)12CH2CH2–), 2.02 (m, –CH2CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2–), 2.51 (2H, –OC6H4CH2–), 2.75–2.90 (m, –CH2CH
CHCH2–), 2.51 (2H, –OC6H4CH2–), 2.75–2.90 (m, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH
CHCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.59 (3H, –OC(
CH–), 3.59 (3H, –OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CH3), 3.90–4.40 (m, 5H, –OCH2CH(OH)CH2OC(O)–), 4.97–5.80 (m, –CH2CH
O)CH3), 3.90–4.40 (m, 5H, –OCH2CH(OH)CH2OC(O)–), 4.97–5.80 (m, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2–), 6.50–6.83 (m, 3H, aromatic), 7.13 (1H, aromatic). FT-IR: 3460 cm−1 (O–H stretching vibration), 3010 cm−1 (C–H vibration of the unsaturated hydrocarbon), 1728 cm−1 (C
CHCH2–), 6.50–6.83 (m, 3H, aromatic), 7.13 (1H, aromatic). FT-IR: 3460 cm−1 (O–H stretching vibration), 3010 cm−1 (C–H vibration of the unsaturated hydrocarbon), 1728 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration (saturated aliphatic ester)), 1257 cm−1 (C(Ar)–O–C asymmetric stretching vibration (m-alkyl phenol)), 1051 cm−1 (C(Ar)–O–C symmetric stretching vibration (m-alkyl phenol)), 775 cm−1 (–CH2– rocking vibration), 721 cm−1 (–(CH2)n–, n > 3; rocking vibration)), 694 cm−1 (aromatic out of plane C–H deformation vibration of meta-substituted benzene).
O stretching vibration (saturated aliphatic ester)), 1257 cm−1 (C(Ar)–O–C asymmetric stretching vibration (m-alkyl phenol)), 1051 cm−1 (C(Ar)–O–C symmetric stretching vibration (m-alkyl phenol)), 775 cm−1 (–CH2– rocking vibration), 721 cm−1 (–(CH2)n–, n > 3; rocking vibration)), 694 cm−1 (aromatic out of plane C–H deformation vibration of meta-substituted benzene).
2.4. Preparation of cross-linked PHM (PHMC) films
10 wt% of polymer solutions in THF were drop casted onto glass or silicon wafer substrates and dried in vacuum overnight. The coatings were irradiated with 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 μW cm−2 UV light (B-100AP ultraviolet lamp, UVP Inc., USA) at a distance of 5 cm for 2 days in air at room temperature to prepare cross-linked PHM (named as PHMC) films.
700 μW cm−2 UV light (B-100AP ultraviolet lamp, UVP Inc., USA) at a distance of 5 cm for 2 days in air at room temperature to prepare cross-linked PHM (named as PHMC) films.
2.5. Gel fraction measurement
The gel fractions of PHMC films were measured by the solvent extraction method.50 The free standing films for the experiments could be obtained by the solution casting method by dropping the polymer solutions on silicon wafers treated with poly(4-vinyl phenol) (PVP) by spin-coating method (3000 rpm, 30 s), and then cross-linked by UV irradiation for 2 days. After the UV cross-linking process, the samples were soaked in excess methanol until cross-linked films were detached from silicon substrates. The films were washed with methanol repeatedly to remove any solvents and the PVP remained, and then dried at 80 °C under vacuum. The dry films were weighted (W1), and then they were soaked and refluxed in excess THF for 24 h. The solvent was changed frequently until extraction was completed. The films were then repeatedly washed with distilled water and dried at 80 °C under vacuum for 24 h until constant weight (W2) was obtained. The gel fractions were calculated as follows:| Gel fraction (%) = W2/W1 × 100 | (1) |
2.6. Antibacterial test
Escherichia coli (E. coli; ATCC 8739) was used for the antibacterial test. To prepare the bacteria suspension, E. coli was cultured in the corresponding broth solutions at 37 °C for 18 h. A representative colony was lifted off with a platinum loop, placed in 30 mL of nutrient broth, and incubated with shaking at 37 °C for 18 h. After washing twice with phosphate buffer saline (PBS), they were re-suspended in PBS to yield 1 × 106 colony forming unit (CFU) per mL.19 Bacterial cell concentration was estimated by measuring the absorbance of cell dispersions at 600 nm and referenced to a standard calibration curve. An optical density of 0.1 at 600 nm is approximately equivalent to 108 cells per mL.20 To evaluate the antibacterial activity of polymer films, 0.1 mL of the bacterial suspension was dropped onto the surfaces of the polymer films (2 cm × 2 cm) located in Petri dish and the films were covered using OHP films having the same size to ensure full contact. After 24 h at 25 °C, 0.9 mL of PBS was poured into the Petri dishes that contain the samples. After vigorous shaking to detach adherent cells from the films, the solution mixture was transferred to micro tube. The resulting solution was serially diluted and then 0.1 mL of each diluent was spread onto the agar plates. Viable microbial colonies were counted after incubated for 18 h at 37 °C. Each test was repeated at least three times. Bacterial inhibition rate was calculated as follows:| Bacterial inhibition rate (%) = 100 × (N0 − Ni)/N0 | (2) |
2.7. Characterization
The chemical structure of the monomers and polymers was characterized by 1H NMR spectroscopy (ZEOL LNM-LA 300, 300 MHz) using CDCl3 as a solvent. IR spectra were recorded on Nicolet 6700 spectrophotometer (Thermo Scientific, USA) using Attenuated Total Reflectance (ATR) equipment (FT-IR/ATR). Molecular weight (Mn, Mw) and PDI were analyzed by gel permeation chromatography (GPC). Relative molecular weight measurements were carried out using a Waters 515 HPLC pump equipped with three columns including PLgel 5.0 μm guard, MIXED-C, and MIXED-D from Polymer Laboratories at 35 °C in series with a Viscotec LR125 laser refractometer. The system with a refractive index (RI) detector was calibrated using polystyrene standards from Polymer Laboratories. HPLC grade THF (J. T. Baker) was used as an eluent at a flow rate of 1.0 mL min−1 at 35 °C. Thermal stability of polymers was investigated by thermal gravimetric analysis (TGA) using TA Instruments TGA Q-5000IR under both nitrogen (N2) and air atmospheres. The samples were first heated to 120 °C and stayed for 10 min at 120 °C, and then heated to 600 °C at a heating rate of 10 °C min−1. Mass spectra were recorded by EI mode at 70 eV with a JEOL JMS-700 mass spectrometer. Thermal transition of polymers was analyzed by differential scanning calorimetry (DSC) with TA Instruments DSC-Q1000 under N2 atmosphere. Samples with a typical mass of 3–7 mg were encapsulated in sealed aluminum pans. The samples except PHM100 were first heated to 80 °C and then quenched to −30 °C, followed by a second heating scan from −30 to 220 °C at a heating rate of 5 °C min−1. Thermal transition of PHM100 was analyzed by the same condition except quenching temperature (−50 °C) and second heating scan range (from −50 to 220 °C). UV-Vis spectra were measured by Agilent 8453 UV-Visible Spectrometer at room temperature. Individual film thickness of cross-linked PHM (PHMC) and PMMA was determined using a micrometer MDC-25PJ (Mitutoyo micrometer, Tokyo, Japan). Gloss value of the polymer film was measured by Gloss master 60° (Sheen instruments, England) gloss meter. Microindentation measurements were performed at room temperature using a Leitz tester and a Vickers diamond indenter. Loads of 5 mN were applied for 20 s and subsequently released to measure the residual area of indentation. Martens hardness values are calculated as follows:| HM = P/(26.43hmax2) | (3) |
3. Results and discussion
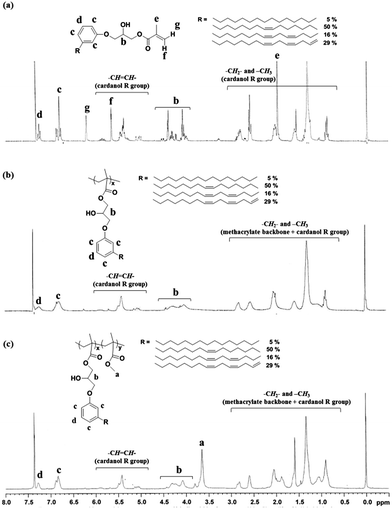
2-Hydroxy-3-cardanylpropyl methacrylate (HCPM) was prepared from the reaction of a renewable resource, cardanol, with glycidyl methacrylate having two reactive functional groups, epoxy and methacrylate groups, in the presence of a base catalyst, KOH (Scheme 1(a)).23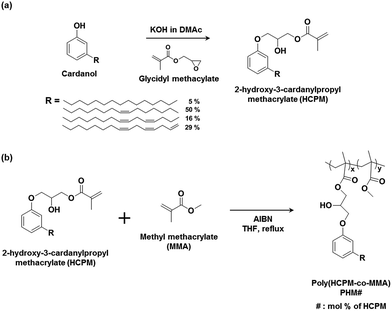 | ||
| Scheme 1 Synthetic route of (a) 2-hydroxy-3-cardanolpropyl methacrylate (HCPM) and (b) poly(HCPM-r-MMA) (PHMs). | ||
The chemical structure of HCPM was confirmed by 1H NMR and FT-IR/ATR. Characteristic absorption peaks of methacrylate protons were observed at 1.97 (–OC(O)C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.62 and 6.26 (–OC(O)C(CH3)
CH2), 5.62 and 6.26 (–OC(O)C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) ppm from the 1H NMR spectrum and the characteristic carbonyl stretching frequency of an ester group appeared at 1720 cm−1 in the FT-IR/ATR (Fig. 1(a) and S1†). It was also found that the unsaturated hydrocarbon in the cardanol moiety was intact after the reaction of cardanol with glycidyl methacrylate, confirmed by a comparison of the peak intensities from the double bonds and other moieties in HCPM (Fig. 1). The PHM#s (where # is the molar compositional ratio of HCPM in polymers) having 0, 10, 47 and 100 mol% of HCPM were synthesized via free radical polymerization using HCPM and MMA as co-monomers with AIBN as the initiator (Scheme 1(b)). Considering the many potential applications of PMMA as coating materials,18 MMA was selected as the co-monomer for the preparation of polymers containing the cardanol moieties. Fig. 1(b) and (c) show 1H NMR spectra and assignment of the respective proton peaks of PHMs. The disappearance of the methacrylate double bond peaks at 5.62 and 6.26 (–OC(O)C(CH3)
CH2) ppm from the 1H NMR spectrum and the characteristic carbonyl stretching frequency of an ester group appeared at 1720 cm−1 in the FT-IR/ATR (Fig. 1(a) and S1†). It was also found that the unsaturated hydrocarbon in the cardanol moiety was intact after the reaction of cardanol with glycidyl methacrylate, confirmed by a comparison of the peak intensities from the double bonds and other moieties in HCPM (Fig. 1). The PHM#s (where # is the molar compositional ratio of HCPM in polymers) having 0, 10, 47 and 100 mol% of HCPM were synthesized via free radical polymerization using HCPM and MMA as co-monomers with AIBN as the initiator (Scheme 1(b)). Considering the many potential applications of PMMA as coating materials,18 MMA was selected as the co-monomer for the preparation of polymers containing the cardanol moieties. Fig. 1(b) and (c) show 1H NMR spectra and assignment of the respective proton peaks of PHMs. The disappearance of the methacrylate double bond peaks at 5.62 and 6.26 (–OC(O)C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) ppm in HCPM and the appearance of the broad peaks at 1.20–1.90 ppm for the aliphatic –CH2– groups in the cardanol moiety of HCPM shown in Fig. 1(b) indicate the successful synthesis of PHM100, the homopolymer containing only HCPM monomeric units. The integral of peaks at 4.97–5.80 ppm, which originated from the unsaturated hydrocarbon chain, was not changed after the polymerization compared to those originated from other groups in HCPM, indicating that the double bonds in the cardanol moiety are not involved in the free radical polymerization reaction. All of the corresponding peaks of PHM100 and PMMA (data not shown) were observed in the spectrum of PHM47 (Fig. 1(c)), confirming the formation of the copolymer. The HCPM content of PHM47 was calculated by comparing the integral of the singlet at 3.59 ppm (a, three protons) with the integral of the peak of the cardanol moiety at 6.8 ppm (c, one proton); it was 47 mol% within experimental error. The contents of HCPM in other PHMs were calculated in similar manners; they are listed in Table 1. The contents of HCPM in copolymers were found to be close to the feeding ratios of HCPM in the polymerization, indicating that the reactivity of HCPM is close to that of MMA although the HCPM contains a long alkyl chain in the cardanol moiety.
CH2) ppm in HCPM and the appearance of the broad peaks at 1.20–1.90 ppm for the aliphatic –CH2– groups in the cardanol moiety of HCPM shown in Fig. 1(b) indicate the successful synthesis of PHM100, the homopolymer containing only HCPM monomeric units. The integral of peaks at 4.97–5.80 ppm, which originated from the unsaturated hydrocarbon chain, was not changed after the polymerization compared to those originated from other groups in HCPM, indicating that the double bonds in the cardanol moiety are not involved in the free radical polymerization reaction. All of the corresponding peaks of PHM100 and PMMA (data not shown) were observed in the spectrum of PHM47 (Fig. 1(c)), confirming the formation of the copolymer. The HCPM content of PHM47 was calculated by comparing the integral of the singlet at 3.59 ppm (a, three protons) with the integral of the peak of the cardanol moiety at 6.8 ppm (c, one proton); it was 47 mol% within experimental error. The contents of HCPM in other PHMs were calculated in similar manners; they are listed in Table 1. The contents of HCPM in copolymers were found to be close to the feeding ratios of HCPM in the polymerization, indicating that the reactivity of HCPM is close to that of MMA although the HCPM contains a long alkyl chain in the cardanol moiety.
We intentionally prepared PHMs and PMMA having relatively low molecular weights in the range of about 5000 to 8000 of number average molecular weight (Mn) using relatively large amount of initiator (about 5 mol% of the monomers) because polymers having these molecular weights are widely used for a variety of coating applications including paints and lithography.24–26 The thermal stability of the PHMs was examined by thermal gravimetric analysis (TGA) under both nitrogen and air atmospheres. Thermal decomposition temperatures for 10 wt% loss (Td,10%) and char yields obtained from the TGA curves (Fig. S2 and S3†) are summarized in Table 2. In both atmosphere, the decomposition temperature increases with increasing the content of HCPM in PHMs. For example, Td,10% in nitrogen condition of PMMA and PHM100 were 263 and 371 °C, respectively. Thus, the increase of HCPM content in PHMs provides additional stabilization energy by cohesive interactions between long alkyl chains in cardanol moieties. The increase in thermal stability of poly(acrylate) by the incorporation of cardanol moieties was reported previously by others.7
| PHM100 | PHM47 | PHM10 | PMMA | ||
|---|---|---|---|---|---|
| a Obtained by DSC equipped with RCS at a heating rate of 5 °C min−1.b The decomposition temperature (Td,10%) is defined as 10 wt% loss.c The char yield at 600 °C. | |||||
| Tga (°C) | −4.60 | 14.0 | 21.4 | 93.9 | |
| Td,10%b (°C) | Under N2 | 371 | 339 | 305 | 263 |
| Under air | 333 | 312 | 221 | 206 | |
| Char yieldc (%) | Under N2 | 1.9 | 1.1 | 0.0 | 0.0 |
| Under air | 2.4 | 0.0 | 0.0 | 0.0 | |
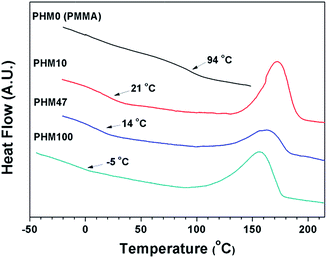
Fig. 2 shows differential scanning calorimeter (DSC) heating curves of the PHMs. Since the PHMs were prepared by a free radical polymerization, they did not show any melting behavior; only glass transition temperatures (Tg) were observed. The amorphous structure of PHMs could be also confirmed from XRD results (Fig. S4†). The value of Tg decreased from 93.9 °C to −4.60 °C as the content of HCPM increased from 0 mol% to 100 mol%. Therefore, the long hydrocarbon chains in the cardanol moiety decrease the glass transition temperatures because they act as plasticizer, preventing close packing between the rigid polymer backbones.27,28 Interestingly, all the PHMs showed a broad exothermic peak at ∼163 °C. It is well-known that drying oils containing unsaturated double bonds can be cross-linked in air by an autoxidation mechanism.29 Since the cardanol moieties in PHMs have a double bond structure, the exothermic peak at 163 °C should be arisen from cross-linking reactions during the DSC heating scan. It is also known that cross-linking reactions in drying oils are accelerated by heating and/or UV irradiation.6
To confirm the cross-linking reactions of the double bonds in the cardanol moieties, FT-IR/ATR spectra of PHM100 film on glass substrate were monitored during the UV irradiation up to 2 days. The long irradiation time, 2 days, for the preparation of the cross-linked PHM (PHMC) films could be much decreased by adding small amount of curing agents.4 While we did not add such curing agents because they can affect the chemical and physical properties especially the antibacterial property of the polymers. The cross-linking reactions originated from the unsaturated bonds could be either monitored by the intensity change of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching peak at 1600–1630 cm−1 or C–H stretching vibration peak at 3010 cm−1 from the unsaturated hydrocarbon. However, since the C
C stretching peak at 1600–1630 cm−1 or C–H stretching vibration peak at 3010 cm−1 from the unsaturated hydrocarbon. However, since the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching peak overlaps with those from benzene ring of HCPM moiety, this change could not be used as shown in Fig. S5,† while the C–H peak does not overlap with any other peaks and its intensity was found to decrease with time, and disappeared completely after 2 days as shown in Fig. 3. The formation of cross-linked structures by UV irradiation could be also confirmed from the change of the sticky state of PHM100 (Tg = −4.60 °C) to a stable and glossy state. Also, the exothermic peak observed on the DSC trace of PHM100 disappeared and the Tg of PHM100 shifted from −4.60 °C to 13 °C after UV irradiation (Fig. S6†). Other PHMs having smaller contents of cardanol moieties also showed similar cross-linking behavior upon UV irradiation. The degree of cross-linking of the PHMC films could be estimated by measuring the gel fraction values.30 The PHMC films exhibited the gel fraction values in the range from 75.5 to 94.3%, indicating that they are highly cross-linked by the UV irradiation (Table 3). In addition, it clearly shows that the larger the content of HCPM moiety, the larger the cross-linking density as expected.
C stretching peak overlaps with those from benzene ring of HCPM moiety, this change could not be used as shown in Fig. S5,† while the C–H peak does not overlap with any other peaks and its intensity was found to decrease with time, and disappeared completely after 2 days as shown in Fig. 3. The formation of cross-linked structures by UV irradiation could be also confirmed from the change of the sticky state of PHM100 (Tg = −4.60 °C) to a stable and glossy state. Also, the exothermic peak observed on the DSC trace of PHM100 disappeared and the Tg of PHM100 shifted from −4.60 °C to 13 °C after UV irradiation (Fig. S6†). Other PHMs having smaller contents of cardanol moieties also showed similar cross-linking behavior upon UV irradiation. The degree of cross-linking of the PHMC films could be estimated by measuring the gel fraction values.30 The PHMC films exhibited the gel fraction values in the range from 75.5 to 94.3%, indicating that they are highly cross-linked by the UV irradiation (Table 3). In addition, it clearly shows that the larger the content of HCPM moiety, the larger the cross-linking density as expected.
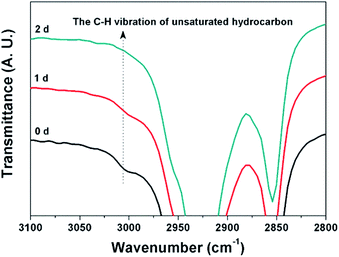 | ||
| Fig. 3 FT-IR/ATR spectra of PHM100 film in the high frequency region after UV irradiation for 1 and 2 days. | ||
| Samplesa | Density (g cm−3) | Microindentation analysis | Gloss (units) | RMS (nm) | Gel fraction (%) | |
|---|---|---|---|---|---|---|
| Martens hardness (HM) (N mm−2) | Maximum penetration depth (hmax) (μm) | |||||
| a Samples were coated onto silicon wafers.b UV was irradiated for 2 days. | ||||||
| PHM100Cb | 0.67 | 114 | 1.88 | 103 | 0.77 | 94.3 |
| PHM47Cb | 0.80 | 133 | 1.73 | 96 | 1.15 | 88.6 |
| PHM10Cb | 0.99 | 175 | 1.05 | 101 | 1.17 | 75.5 |
| PMMA | 0.97 | — | — | 97 | 1.35 | — |
To investigate possible use for surface coating applications, optical and mechanical properties of PHMC films were evaluated. For a quantitative analysis of transparency, UV-vis spectra of PHMC films were measured. All the PHMC films show high transmittance in the visual light regions (Fig. 4). Since PMMA coating prepared on glass substrate was easily detached and broken into small fragments as shown in inset of Fig. 4, reproducible UV-vis spectra of PMMA film could not be obtained because the number average molecular weight of the PMMA in this study is only 7100, which is much smaller than the commercialized PMMA for other applications.31 It is clear that the physical strength of PMMA with Mn of 7100 is not sufficient to form a physically stable film, while PHMCs having similar molecular weights can form transparent and ductile films on glass substrate because the side chains are cross-linked to form physically stable films and also possibly their Tgs are lower than that of PMMA. The inset in Fig. 4 shows the high transparency of the cross-linked PHM (PHMC) films on the glass substrates prepared using the drop casting method. Gloss, an important parameter indicating the visual appearance of an object, is an optical property describing the ability of a surface to reflect light into the specular direction.32 Generally, the gloss values of materials are affected by various factors such as refractive index of the materials, the angle of incident light, and the surface topography.33 Among them, the effect of surface topography on the gloss value can be determined using Rayleigh criteria.34–37
h < λ/8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (4) |
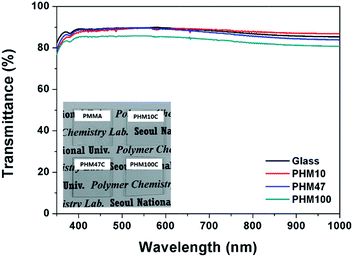 | ||
| Fig. 4 UV-vis spectra of cross-linked PHM (PHMC) films. Inset is photograph of the PHMC films prepared by a solution casting method. | ||
The RMS values of surface roughness for all PHMC films measured using atomic force microscopy (AFM) were found to be smaller than 2.3 nm (Table 3). Therefore, the effect of surface roughness on the gloss value could be ignored in this study (Fig. S8†). Furthermore, the gloss values of PHMC films are comparable to that of PMMA, indicating that the introduction of HCPM into the polymer did not change the gloss properties of PMMA, the well-known glossy polymer.39 The Martens hardness (HM) values of PHMC films were found to decrease with increasing HCPM content in the polymers (Table 3). Since the film thickness values of the PHMC films were ∼110 μm on average and the penetration depth (hmax) in our microindentation study was smaller than 10 μm, the effect of substrate on HM values of the samples could be ignored.40 Then, the change in the HM values in the polymer should only originate from the content of HCPM. An increase in the HCPM content in PHMCs can increase the cross-linking density, because the double bonds, the cross-linking sites, are located in the long hydrocarbon chains in cardanol moieties of HCPM. In many polymer systems, an increase in cross-linking density increases the hardness of the cross-linked polymers.41 However, in our case, the increase of HCPM content can also increase the free volume of the polymers, as estimated by the Tg values of PHMC where the Tg values of PHMC are much smaller than that of PMMA (Fig. S6†).42,43 The larger free volume of the cross-linked PHMCs could also be estimated from the microscopic density of PHMCs. When the content of HCPM was larger than 47 mol%, the density of the PHMC was found to be smaller than that of PMMA (Table 3). However the density of PHM10C having 10 mol% HCPM was found to be slightly larger than that of PMMA. Possibly, small amounts of the more flexible monomeric unit incorporated into the copolymers can increase the density by forming more compact structures by the two monomers, as reported by others.44,45
Although the introduction of HCPM into the polymers decreased the surface hardness of the PHMC films, it can increase the film stability of the polymer. For example, in PMMA without any HCPM moieties and cross-linked structures, HM values could not be obtained because of its brittle properties. However, by the addition of only 10 mol% of HCPM units into PMMA, we could obtain very stable and glossy polymer films having a cross-linked structure formed through UV irradiation. Furthermore, the HCPM moieties in both linear PHMs and cross-linked PHMs were found to impart the bactericidal activity.
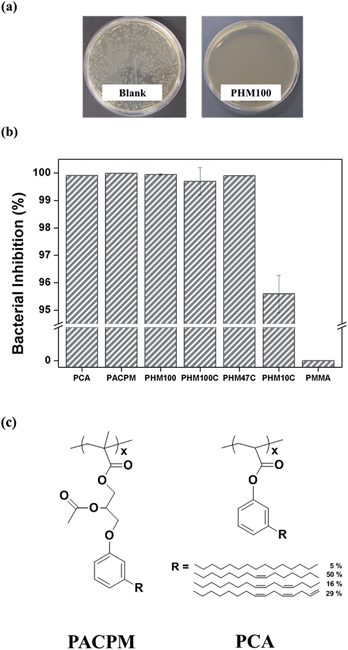
Antibacterial tests of PHM100 and PHMC films were conducted against Escherichia coli (E. coli) using a film-attached method. Each bacteria solution (106 CFU mL−1) was contacted with the polymer films and bare silicon wafer as a control at 25 °C for 24 h. After diluting with phosphate-buffered saline (PBS), aliquots of each sample solutions were spread on agar plates and incubated at 37 °C for 18 h. Fig. 5(a) shows the photographic results of antibacterial tests of blank and PHM100 film, respectively. The numbers of bacterial colonies decreased markedly after contact with the PHM100 film for 24 h compared with the blank sample. The calculated bacterial inhibition rate against E. coli obtained using eqn (2) shows that PHM100 has high antibacterial activity, ∼99.95%. Therefore, it is demonstrated that the original antibacterial properties of cardanol are maintained, although the hydroxyl group of cardanol was reacted with glycidyl methacrylate and then polymerized by a free radical mechanism. One might assume that the antibacterial properties of PHM100 originate from possibly small amount of cardanol remaining in the polymers. Since we purified the polymers solutions in THF by precipitating into H2O/MeOH mixture several times, we believe that all the cardanols were removed. Also, we could not observe any cardanol peaks from GPC and other experiments.
Long amphiphilic chains containing cationic moieties such as ammonium or phosphonium groups are the most well-known chemical structures for the polymers showing the bactericidal property because such amphiphilic moieties can interact with bacteria membrane by both electrostatic and hydrophobic interactions resulting the destruction of the cell membrane structures.51–55 On the contrary, PHMs and PHMC do not have such distinct amphiphilic moieties, although the hydroxyl group and the unsaturated hydrocarbon chain in the side chain are somewhat hydrophilic and hydrophobic, respectively. Therefore to further investigate the effects of structural variation of the cardanol moieties on the bactericidal properties, we intentionally synthesized poly(2-acetoxy-3-cardanylpropyl methacrylate) (PACPM) and poly(cardanyl acrylate) (PCA). Their chemical structures are shown in Fig. 5(c) and the detailed synthetic procedures for these polymers are explained in the ESI.† PACPM and PCA also showed very high antibacterial activity, ∼99.90%. Therefore, cardanol moieties connected to the (meth)acrylic polymers by the 2-hydroxyl propoxy (PHM100), by the 2-acetoxypropoxy (PACPM), and by the ester (PCA) groups can show the same high antibacterial properties. The antibacterial properties of PHMs were found to be maintained after the double bonds on the linear hydrocarbon chains in the cardanol were reacted with each other to form the cross-linked structures. Although most of the double bonds disappeared after the UV irradiation, as shown in the FT-IR/ATR spectra (Fig. 3), PHM100C and PHM47C having 100 and 47 mol% of HCPM units, respectively, also showed almost 99% bactericidal activity. Therefore, the changes in the double bonds in the saturated hydrocarbon structures apparently do not affect the bactericidal properties. The less effective bactericidal properties of PHM10C (95.59%) may have been due to the small content of HCPM units.
4. Conclusions
A series of cardanol-containing polymers (PHMs) were prepared by the radical polymerization of 2-hydroxy-3-cardanylpropyl methacrylate (HCPM) and methyl methacrylate (MMA) as monomers. The thermal and physical stabilities of the brittle PMMA were found to be greatly improved by the incorporation of only 10 mol% of HCPM units in the polymers, and they could be further improved through UV irradiation to form flexible, transparent, and glossy cross-linked PHM (PHMC) films. Both PHM and PHMC films showed excellent antibacterial properties, indicating that the double bond structures in the cardanol moieties do not affect the bactericidal properties. Furthermore, other acrylate polymers having cardanol moieties, such as poly(cardanyl acrylate) and poly(2-acetoxy-3-cardanylpropyl methacrylate) also showed the similarly excellent antibacterial properties, indicating that the connecting groups did not diminish the original bactericidal properties of the cardanol. To the best of our knowledge, this is the first report of the systematic study on the antibacterial properties of cardanol-containing polymers. We believe that these cardanol-containing polymers could be promising candidates for many surface coating applications due to their good thermal, optical, and antibacterial properties as well as their cross-linkability.Acknowledgements
This research was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2010-C1AAA01-0029061).Notes and references
- M. Höök, S. Davidsson, S. Johansson and X. Tang, Philos. Trans. R. Soc., A, 2014, 372, 20120448 CrossRef PubMed.
- R. Heinberg, The Oil Depletion Protocol: A Plan to Avert Oil Wars, Terrorism and Economic Collapse, New Society Publishers, 2013 Search PubMed.
- R. W. Bentley, Energy Policy, 2002, 30, 189 CrossRef.
- A. Kumar, P. K. Vemula, P. M. Ajayan and G. John, Nat. Mater., 2008, 7, 236 CrossRef CAS PubMed.
- R. Ikeda, H. Tanaka, H. Uyama and S. Kobayashi, Macromol. Rapid Commun., 2000, 21, 496 CrossRef CAS.
- G. John and C. Pillai, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 1069 CrossRef CAS PubMed.
- K. I. Suresh and M. Jaikrishna, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5953 CrossRef CAS PubMed.
- J.-K. Francis Suh and H. W. Matthew, Biomaterials, 2000, 21, 2589 CrossRef CAS.
- F. Li, M. Hanson and R. Larock, Polymer, 2001, 42, 1567 CrossRef CAS.
- J. Lunt, Polym. Degrad. Stab., 1998, 59, 145 CrossRef CAS.
- D. Lomonaco, G. M. Pinheiro Santiago, Y. S. Ferreira, Â. M. Campos Arriaga, S. E. Mazzetto, G. Mele and G. Vasapollo, Green Chem., 2009, 11, 31 RSC.
- P. A. Mahanwar and D. D. Kale, J. Appl. Polym. Sci., 1996, 61, 2107 CrossRef CAS.
- P. Peungjitton, P. Sangvanich, S. Pornpakakul, A. Petsom and S. Roengsumran, J. Surfactants Deterg., 2009, 12, 85 CrossRef CAS PubMed.
- M. Himejima and I. Kubo, J. Agric. Food Chem., 1991, 39, 418 CrossRef CAS.
- Y. H. Kim, E. S. An, B. K. Song, D. S. Kim and R. Chelikani, Biotechnol. Lett., 2003, 25, 1521 CrossRef CAS.
- A. R. R. Menon, C. K. S. Pillai and A. G. Mathew, J. Sci. Ind. Res., 1985, 44, 324 CAS.
- R. Ikeda, H. Tanaka, H. Uyama and S. Kobayashi, Polymer, 2002, 43, 3475 CrossRef CAS.
- W. Tanglumlert, P. Prasassarakich, P. Supaphol and S. Wongkasemjit, Surf. Coat. Technol., 2006, 200, 2784 CrossRef CAS PubMed.
- Z. Cao and Y. Sun, ACS Appl. Mater. Interfaces, 2009, 1, 494 CAS.
- N. Bordenave, S. Grelier and V. Coma, Biomacromolecules, 2010, 11, 88 CrossRef CAS PubMed.
- W.-R. Li, X.-B. Xie, Q.-S. Shi, H.-Y. Zeng, O.-Y. You-Sheng and Y.-B. Chen, Appl. Microbiol. Biotechnol., 2010, 85, 1115 CrossRef CAS PubMed.
- L. E. Seitzman, J. Mater. Res., 1998, 13, 2936 CrossRef CAS.
- A. Peutzfeldt, Eur. J. Oral Sci., 1997, 105, 97 CrossRef CAS PubMed.
- H.-S. Sohn, D.-G. Kim, A. Lee, J.-W. Lee, J.-S. Kim, J.-H. Kim and J.-C. Lee, J. Appl. Polym. Sci., 2012, 125, 344 CrossRef CAS PubMed.
- H.-S. Sohn, S.-H. Cha, W.-K. Lee, D.-G. Kim, H.-J. Yun, M.-S. Kim, B.-D. Kim, Y.-H. Kim, J.-W. Lee, J.-S. Kim, D.-B. Kim, J.-H. Kim and J.-C. Lee, Macromol. Res., 2011, 19, 722 CrossRef CAS.
- T. Takayanagi and M. Yamabe, Prog. Org. Coat., 2000, 40, 185 CrossRef CAS.
- J.-C. Lee, S.-H. Han, S.-H. Cha, S.-Y. Park and B. L. Farmer, Polymer, 2003, 44, 7413 CrossRef CAS PubMed.
- H. Bhunia, R. Jana, A. Basak, S. Lenka and G. Nando, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 391 CrossRef CAS.
- R. T. Holman, Prog. Chem. Fats Other Lipids, 1954, 2, 51 CrossRef CAS.
- L. E. Nielsen, J. Macromol. Sci., Polym. Rev., 1969, 3, 69 CrossRef CAS.
- P. Gupta, C. Elkins, T. E. Long and G. L. Wilkes, Polymer, 2005, 46, 4799 CrossRef CAS PubMed.
- M. Lindstrand, Gloss: Measurement, Characterization and Visualization-in the Light of Visual Evaluation, Citeseer, 2002.
- S. Wu, M. T. Sears and M. D. Soucek, Prog. Org. Coat., 1999, 36, 89 CrossRef CAS.
- L. A. Simpson, Prog. Org. Coat., 1978, 6, 1 CrossRef CAS.
- R. Rowe, J. Pharm. Pharmacol., 1985, 37, 761 CrossRef CAS PubMed.
- G. H. Meeten, Optical Properties of Polymers, Elsevier Applied Science Publishers, 1986 Search PubMed.
- J. C. Zwinkels and M. Noel, Surf. Coat. Int., 1995, 78, 512 Search PubMed.
- T. Trezza and J. Krochta, J. Appl. Polym. Sci., 2001, 79, 2221 CrossRef CAS.
- J. Wolfe and H. Mark, Encycl. Polym. Sci. Technol., Wiley, New York, 1988, vol. 11, p. 601 Search PubMed.
- K. Geng, F. Yang, T. Druffel and E. A. Grulke, Polymer, 2005, 46, 11768 CrossRef CAS PubMed.
- H. Kamogawa and T. Sekiya, J. Polym. Sci., 1961, 50, 211 CrossRef CAS PubMed.
- D.-G. Kim, H.-S. Sohn, S.-K. Kim, A. Lee and J.-C. Lee, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3618 CrossRef CAS PubMed.
- S.-K. Kim, D.-G. Kim, A. Lee, H.-S. Sohn, J. J. Wie, N. A. Nguyen, M. E. Mackay and J.-C. Lee, Macromolecules, 2012, 45, 9347 CrossRef CAS.
- C. C. McDowell, B. D. Freeman, G. W. McNeely, M. I. Haider and A. J. Hill, J. Polym. Sci., Part B: Polym. Phys., 1998, 36, 2981 CrossRef CAS.
- S. Pragliola, G. Cavallo, P. Longo and V. Venditto, Polymer, 2013, 54, 3767 CrossRef CAS PubMed.
- E. R. Kenawy, F. I. Abdel-Hay, A. E. R. R. El-Shanshoury and M. H. El-Newehy, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 2384 CrossRef CAS PubMed.
- J. Ahn, E.-H. Sohn, S. H. Bang, J. Kang, T. Kim, H. Hong, S.-E. Kim, B.-S. Kim, J. Yoon and J.-C. Lee, Macromol. Res., 2014, 22, 337 CrossRef CAS PubMed.
- K. Choi, S.-E. Kim, J.-Y. Kim, J. Yoon and J.-C. Lee, J. Nanosci. Nanotechnol., 2008, 8, 5360 CrossRef CAS PubMed.
- K. Choi, M.-J. Nam, J. Y. Kim, J. Yoon and J.-C. Lee, Macromol. Res., 2011, 19, 1227 CrossRef CAS PubMed.
- S.-K. Kim, K.-H. Kim, J. O. Park, K. Kim, T. Ko, S.-W. Choi, C. Pak, H. Chang and J.-C. Lee, J. Power Sources, 2013, 226, 346 CrossRef CAS PubMed.
- E.-R. Kenawy, S. Worley and R. Broughton, Biomacromolecules, 2007, 8, 1359 CrossRef CAS PubMed.
- L. Timofeeva and N. Kleshcheva, Appl. Microbiol. Biotechnol., 2011, 89, 475 CrossRef CAS PubMed.
- M. Zasloff, Nature, 2002, 415, 389 CrossRef CAS PubMed.
- M. Van Loosdrecht, J. Lyklema, W. Norde, G. Schraa and A. Zehnder, Appl. Environ. Microbiol., 1987, 53, 1893 CAS.
- J. S. Dickson and M. Koohmaraie, Appl. Environ. Microbiol., 1989, 55, 832 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06223j |
| ‡ These authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2014 |