Toughening mechanism behind intriguing stress–strain curves in tensile tests of highly enhanced compatibilization of biodegradable poly(lactic acid)/poly(3-hydroxybutyrate-co-4-hydroxybutyrate) blends
Abstract
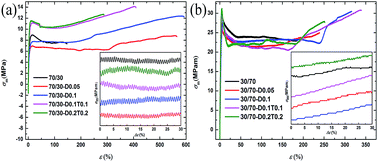
Highly enhanced compatibilization of biosourced and biodegradable polylactide (PLA) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P(3HB-co-4HB)) blends were successfully prepared by reactive melt compounding. Large shifts towards each other in terms of glass transition temperatures, a considerable reduction in the dispersed phase particle size and a significant increase in the interfacial adhesion between the PLA and P(3HB-co-4HB) phases were observed after compatibilization. In addition, chain branches occurred during the branching reaction decreased the crystallization ability of PLA, while crosslinks formed in the crosslinking reaction enhanced the crystallization ability of PLA on a large scale. Moreover, the blends exhibited a remarkable improvement of rheological properties of melt state when compared with that of blank PLA/P(3HB-co-4HB) blends. Upon increasing the content of the crosslinking agent, dicumyl peroxide (DCP), the blends showed increased yield tensile strength, modulus, and elongation at break. However, when DCP cooperated with triallyl isocyanurate (TAIC), the elongation at break decreased because the crosslinking network limited the mobility of the polymer chains to deform under a tensile load. Most notably, two typical and different kinds of growth of stress–strain curves were observed, and for the first time we demonstrated the toughening mechanism behind it in detail. Furthermore, SEM images of the fracture surfaces of the blends confirmed the toughening mechanism and that plastic deformation of the matrix and a debonding process were the two important ways of induced energy dissipation leading to toughened blends.

 Please wait while we load your content...
Please wait while we load your content...