Recent developments in one-pot tandem oxidation process coupling reactions
Vineet Jeena
* and
Ross S. Robinson
Department of Chemistry, University of KwaZulu-Natal, Scottsville, Pietermaritzburg, South Africa. E-mail: Jeenav1@ukzn.ac.za
First published on 19th August 2014
Abstract
The tandem oxidation process (TOP) constitutes a novel approach to synthetic organic chemistry. This procedure involves the oxidation of the alcohol to the aldehyde which is trapped in situ by a nucleophile leading to a variety of synthetically useful compounds. In recent times, many research groups have focused their attention on developing new approaches to the one-pot coupling process. In this review, we highlight the latest additions, over the past nine years, to the TOP realm such as the use of novel oxidants, innovative catalysts and alternate reaction conditions. In addition, the introduction of TOP to multicomponent reactions and total syntheses will also be discussed.
1. Introduction
The standard method for the synthesis of organic molecules is a stepwise process involving the addition of various reagents, catalysts and temperature control measures to effect a desired transformation.1 However it would be more advantageous, from a cost and time perspective, if multiple bonds could be formed without the addition of new reagents or catalysts. This type of procedure, commonly referred to as a domino process, derives its name from the game where a row of dominos are lined up. If the first domino is knocked over, the remaining dominos are also knocked over without changing the conditions.2The tandem oxidation process (TOP) has emerged as an innovative and exciting tool in synthetic organic chemistry. This process comprises of mixing of an alcohol, nucleophile and oxidant in a ‘one-pot’ process, whereby the intermediate aldehyde is immediately trapped and elaborated further. TOP is a variation of the domino process as the nucleophile will not react with the alcohol, rather the alcohol must be oxidized to the aldehyde first, which will trigger the subsequent coupling step. This method is characterized by high yields, short reaction times and obvious time and cost benefits. In addition, this methodology is particularly useful when the intermediate aldehyde is volatile or lachrymatory. Since the discovery and development of this research field, a variety of synthetically useful compounds have been prepared and this approach has also been extended to total syntheses. The TOP research area is divided into two main categories, (i) the variation of the oxidant or (ii) the variation of the nucleophile. In recent times, the TOP research area has exploded with a number of innovative and new applications using a range of oxidants and nucleophiles. In this article, recent developments in the tandem oxidation process since the publication of the last major review will be discussed.
2. History of the tandem oxidation process (TOP)
An important milestone towards the development of the TOP was a report by Robert Ireland and co-worker in 1985.3 During synthetic studies towards polyetherionophore antibiotics, several volatile aldehydes were encountered which made their isolation and consequently, their further elaboration difficult. To circumvent this problem, Ireland used the Swern oxidation to produce the aldehyde and, to the crude mixture added the Wittig reagent. This procedure of adding the Wittig reagent to the crude Swern oxidation mixture produced the desired compound in excellent yields. A series of examples as reported by Ireland is illustrated below (Scheme 1).This approach was of vital importance as it allowed for the elaboration of the aldehyde and provided the desired compounds in excellent yields. Despite the benefits of this procedure, there was one obvious caveat: What if the aldehyde is so volatile that it decomposes or polymerizes before the nucleophile is added?
This problem was effectively solved by Huang and co-workers in 1987 while attempting the synthesis of carbon-14-labelled CI-933 1 which has amnesia reversal activity.4 During the synthesis, the unsaturated ester was required which was envisioned to be obtained by the oxidation of the alcohol to the aldehyde and subsequent Wittig reaction. However, despite numerous procedures being attempted, the aldehyde could not be obtained. This obstacle was overcome by mixing the alcohol, Wittig reagent and the Dess–Martin periodinane reagent in a one-pot synthesis. Using this approach, the desired compound was isolated in a satisfactory yield of 78% (Scheme 2). It is also worth mentioning that an alternate approach to access the unsaturated ester from the alcohol required eight synthetic steps with a 20% overall yield. This excellent piece of research remained untouched for 11 years until the Taylor research group established it as a core research area fully examining its scope.
In 1998, the Taylor research group was investigating synthetic routes towards the manumycin family of antibiotics.5–8 A key step of this synthesis involved the oxidation of alcohol 2 to the aldehyde 3, followed by a Wittig coupling reaction to produce the dienoate 4 (Scheme 3).
However, the propensity of the aldehyde to polymerize/oligiomerize upon isolation as well as its lachrymatory nature resulted in low yields of the desired dienoate. When the Ireland procedure (outlined above) was employed, disappointing yields were still observed indicating the volatility of the intermediate aldehyde. Finally, Xudong Wei (a member of the Taylor research group) mixed the alcohol, Wittig reagent and manganese dioxide in a one pot synthesis. Under these conditions, the desired dienoate was formed in a good yield of 78% (Scheme 4).
The Taylor group, named this procedure the tandem oxidation process (TOP) in order to differentiate it from the Ireland procedure. Thus, a captivating field of research had been born and during the coming years, the Taylor group emerged as leaders in this research by exploring its scope and limitations on many systems.
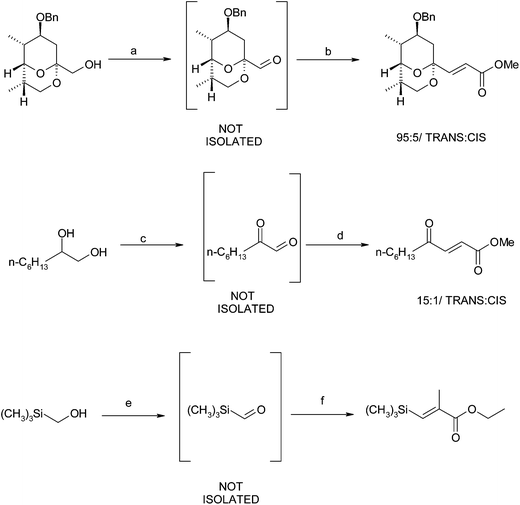
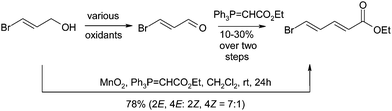
The late 1990's was an exciting time for the tandem oxidation process as a number of other groups also reported their contributions to this field. In addition to Taylor's report, various groups reported a Dess–Martin periodinane,9 BaMnO410 and IBX11 mediated TOPs. The Taylor group however, remained at the forefront of this research and applied their MnO2-mediated TOP approach towards the synthesis of various useful compounds. The majority of these examples have been discussed in the previous review on the tandem oxidation process12 however a visual representation showing a selection of systems examined by the Taylor group is given below.13 The first step involves the oxidation of the alcohol to the aldehyde (Scheme 5) or the keto-aldehyde (Scheme 6) which is trapped and elaborated further.
3. Synthesis of quinoxalines
The synthesis of quinoxalines is synonymous with the tandem oxidation process and every year numerous methods are reported to access this moiety. A clear drawback of the MnO2 mediated TOP is the requirement of 10 equivalents of the oxidant. Thus, research has been directed towards the development of new, environmentally friendly and catalytic oxidants.3.1 K-OMS-2
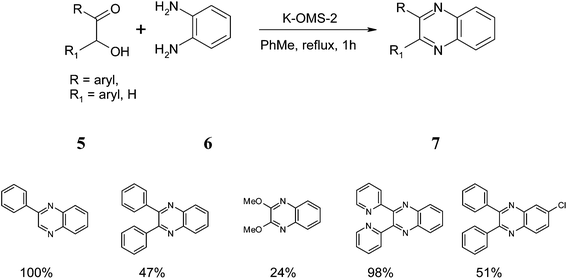
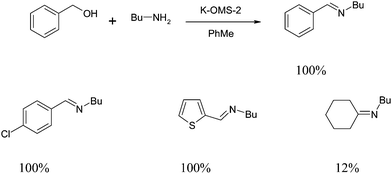
An interesting example of this is the use of manganese dioxide octahedral molecular sieves (K-OMS-2) for the synthesis of quinoxalines. K-OMS-2 is a cryptomelane-type manganese oxide with the composition KMn8O16·nH2O and consists of MnO6 octahedral units.14 These catalysts were prepared using a literature method and found to catalyze the coupling of keto-alcohols 5 and diamines 6 to quinoxalines 7 in 100% selectivity. Using this approach, a series of quinoxaline derivatives were synthesized with varying yields depending on the substrate examined (Scheme 7).15Interestingly, the synthesized catalyst can be reused without any loss in activity as demonstrated in the following table. The catalyst was recovered by simply washing with acetone/methanol and water and heating to 250 °C. However, during the filtration process, a reduced amount of catalyst was recovered and therefore the authors compared the performance of the ‘used’ catalyst and ‘fresh’ catalyst in the same quantities (Table 1).
This result was of utmost importance and is certainly an improvement on the traditional MnO2 oxidation which required 10 equivalents of MnO2.
3.2 Gold
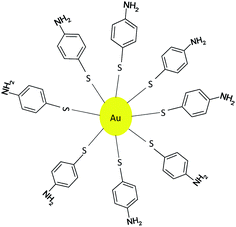
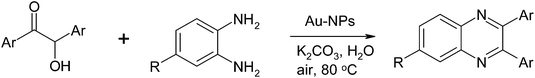
The use of gold nanoparticles (Au-NPs) has also been used in the synthesis of quinoxalines. These 4-aminothiophenol monolayer-coated Au-NPs were prepared in a one pot procedure using 4-aminothiophenol as both the reducing and stabilizing agent.16 The Au-NPs were stabilized by thiolates adsorbed onto the surfaces of the nanoparticles and the self-assembled monolayer of 4-aminothiophenol on the surface of Au-NPs is illustrated below (Fig. 1).An optimization study revealed that 4.0 atom% of the Au-NPs with K2CO3 as a base in an aqueous solvent can effect the conversion of α-hydroxy ketones to afford 1,2-diketones. This observation was extended to the synthesis of quinoxalines by adding a 1,2-diamino nucleophile in a one pot synthesis.17 The use of a Au-NP mediated TOP resulted in the synthesis of quinoxalines in excellent yields as illustrated below (Table 2).
3.3 Copper
The use of copper in TOP is highly desirable as copper is cheap, readily available and environmentally benign. The use of a copper catalyzed oxidative cyclization of α-hydroxy ketones and o-phenylene diamines leading to quinoxalines has also been developed. During the initial studies various sources of copper were examined and CuCl2 was chosen as it provided the desired quinoxalines in highest yield in the shortest reaction time.18 A selection of results obtained is illustrated below (Scheme 8).In subsequent, papers the same authors' reported an improved strategy using a catalytic amount of copper with molecular sieves under an oxygen atmosphere.19 As pointed out by the authors', this recyclable catalytic system is valuable from a green and sustainable chemistry point of view.
3.4 Ruthenium
Another example of a transition metal catalyzed TOP quinoxaline synthesis is the use of a catalytic amount of ruthenium.20 During the optimization step, it was found that a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of alcohol to diamine in combination with potassium hydroxide and benzalacetone provided the desired quinoxaline in the highest yield. This procedure is fascinating as the oxidation and coupling reaction is evaluated on a diol 8 rather than the traditional keto-alcohol (Scheme 9).
1 ratio of alcohol to diamine in combination with potassium hydroxide and benzalacetone provided the desired quinoxaline in the highest yield. This procedure is fascinating as the oxidation and coupling reaction is evaluated on a diol 8 rather than the traditional keto-alcohol (Scheme 9).
3.5 Iron
Having examined gold, copper and ruthenium mediated quinoxaline synthesis; attention is now directed towards an iron mediated reaction. In 2009, Lingaiah and co-workers were interested in an iron promoted TOP quinoxaline synthesis21 and examined the activity of various sources of iron in the coupling reaction of benzoin 9 and o-phenylenediamine 10. Of the iron sources evaluated iron exchanged molybdophosphoric acid (FeMPA), prepared by the reaction of iron nitrate and 12-molybdophosphoric acid, displayed the greatest performance in the coupling reaction. Interestingly, other sources of iron such ferric chloride and ferric nitrate displayed diminished activity as did pure 12-molybdophosphoric acid as demonstrated below (Table 3).A series of quinoxalines synthesized using the FeMPA/O2 system in refluxing CH3CN is illustrated below (Fig. 2).
A related system has also been reported with FeCl3 in combination with morpholine catalyzing the coupling of α-hydroxyketones and 1,2-diamines.22 Using this method, the desired quinoxalines were synthesized in comparable yields to the above iron-mediated system.
3.6 Gallium
The synthesis of quinoxalines using the TOP has also been reported using a gallium mediated procedure.23 The use of Ga(ClO4)3 has been shown to catalyze the synthesis of quinoxalines in excellent yields (Scheme 10).3.7 Solid acid catalyzed quinoxaline synthesis
In our laboratories, we have explored the use of titanium dioxide as part of a photocatalyzed tandem oxidation quinoxaline synthesis.24 Our initial studies were comprised of monitoring the coupling between 2-hydroxyacetophenone 11 and o-phenylenediamine and, using natural sunlight as a source of irradiation, the desired 2-phenylquinoxaline 12 was formed in an excellent yield of 90%. However, the use of natural sunlight suffers from availability and reproducibility limitations and therefore, the above coupling system was optimized using microwave irradiation. Thus, using a TiO2 microwave mediated system, a series of quinoxalines were formed in excellent yields (Scheme 11).Microwave chemistry has emerged as a valuable addition to synthetic organic chemistry with higher yields and shorter reaction times being reported compared to traditional heating methods.25 In addition, the use of specific temperature and pressure controlled scientific reactors has further enhanced this field as they display numerous advantages over multimode commercial microwaves (“kitchen” microwaves).26 In a continuation of MnO2 mediated quinoxalines, Chung and co-workers have reported that under closed vessel microwave conditions, 1 mg of MnO2 can catalyze the synthesis of 2-phenylquinoxaline 12 in 81% in just 1 minute.27 Interestingly, under identical conditions however, with the use of open vessel microwave irradiation, the yield of 2-phenylquinoxaline 12 decreases dramatically to just 19%. One theory proposed, for the observed discrepancies in yield, is that oxygen from the air may be acting as the stoichiometric oxidant under closed vessel conditions.12 In our laboratories, we have shown that just 5 mg of silica gel can also catalyze this reaction (Scheme 12) with yields comparable to the 1 mg MnO2 system.28 We further explored the effect of open or closed vessel microwave conditions and found that a slight increase in pressure, obtained under closed vessel conditions, is responsible for the discrepancies in yields.
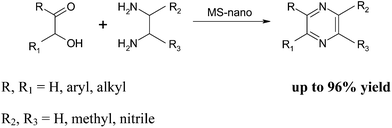
3.8 Synthesis of pyrazines
In continuation of the solid acid catalyzed TOP, the Ghosh research group has reported the use of γ-maghemite silica nanocomposite (MS-nano) in the synthesis of pyrazines.29 The synthesis of the catalyst involved the mixing of FeCl3 and silica gel which was subsequently heated to produce a powder which was used to catalyze the synthesis of pyrazines. Using this approach, a series of pyrazine derivatives were formed in excellent yields (up to 96%) (Scheme 13). However, with the synthesis of specialized catalysts, a recover and re-use study is almost mandatory and the developed catalyst could be re-used up to 10 times without any significant loss in activity.Each of these procedures, for the TOP-mediated quinoxaline synthesis, has a number of positive attributes such as short reaction times and high yields but, in our opinion, the solvent-free silica gel procedure is perhaps the best option. This procedure is relatively inexpensive as it is conducted in the absence of solvent using just 5 mg of silica gel and good to excellent yields are obtained in 10–20 minutes. In particular, the choice of silica gel as a catalyst is attractive as it is readily available in most chemical laboratories and can be used ‘as is’ avoiding the task of preparing complex catalysts.
4. Synthesis of alkenes
The Wittig reaction is an important reaction in organic chemistry as it provides a reliable route to α, β unsaturated esters.30 This reaction, along with the synthesis of quinoxalines, is synonymous with TOP and, since the last review, the Taylor group continued to pursue MnO2-mediated TOP towards Wittig type products.4.1 MnO2
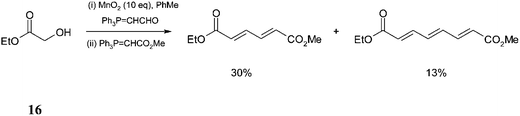
This report, an example of a Wittig–Wittig TOP process was discovered as the Taylor group needed to develop easy access to a range of oxygenated dienoates. The aim was to convert the ketomalonate 13 to a homologated aldehyde 14 using (triphenylphosphoranylidene)acetaldehyde (Trippett's reagent) or its α-methyl analogue and then carry out a second Wittig in situ to produce the desired compounds 15 (Scheme 14).Interestingly, when the above system was applied to an α-hydroxy ester, ethyl glycolate 16 the expected dienoate, albeit in low yield was obtained, as well as the triene resulting from an oxidation triple Wittig sequence31 (Scheme 15). The reaction produced no other by-products so the low yields may be due to the volatility of the products.
The formation of the triene was exciting and consequently, the Taylor group briefly explored its merits by using α-hydroxyacetophenone 17 with MnO2 and 2 equiv. of Trippett's reagent followed by the treatment of Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me which produced the triene in 47% yield along with the diene in 29% yield (Scheme 16).
CHCO2Me which produced the triene in 47% yield along with the diene in 29% yield (Scheme 16).
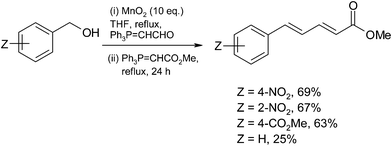
Finally, the tandem oxidation Wittig–Wittig sequence was extended to benzylic alcohols. The reactions were extremely slow at room temperature and 4-nitro benzyl alcohol was examined using various solvents at elevated temperatures and THF was the solvent of choice as it produced the desired compound in optimum yield (THF = 69%, CH2Cl2, Δ, 54%; DMF, 100 °C, 54%; PhMe, Δ, 34%).
The low reactivity of the phosphorane (Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCHO) leads to a limitation of this process and only benzylic alcohols which produce electron deficient benzaldehydes undergo Wittig coupling efficiently as illustrated by the following data. 4-nitro, 2-nitro and 4-carbomethoxy benzyl alcohols gave reasonable yields while benzyl alcohol produced the diene in only 25% yield (Scheme 17).
CHCHO) leads to a limitation of this process and only benzylic alcohols which produce electron deficient benzaldehydes undergo Wittig coupling efficiently as illustrated by the following data. 4-nitro, 2-nitro and 4-carbomethoxy benzyl alcohols gave reasonable yields while benzyl alcohol produced the diene in only 25% yield (Scheme 17).
4.2 Ruthenium
In 2009, Park and co-workers reported a ruthenium catalyzed one-pot oxidation Wittig coupling reaction in the presence of oxygen (Scheme 18).32 This procedure involved the preparation of a [Ru/AlO(OH)] catalyst by mixing ruthenium(III) chloride hydrate, ethanol and Al(sec-OBu)3. The designed method was applied to a series of aliphatic and benzylic alcohols with great success.4.3 Wittig–Diels–Alder multicomponent reaction
The traditional oxidation-Wittig reaction was taken further by conducting a Wittig–Diels–Alder reaction commencing from the alcohol.33 This strategy involved the construction of the dienophile component through a tandem oxidation-Wittig reaction, which subsequently undergoes a Diels–Alder cycloaddition in the presence of a diene. Manganese dioxide and Pyridinium Chlorochromate (PCC) were used as oxidants and under both conditions the highly functionalized cyclic structures were formed in good yields (Scheme 19).The TOP-mediated Wittig has, surprisingly, not received as much attention over recent years when compared to the TOP-mediated quinoxaline synthesis. Of the two examples, both MnO2 and ruthenium mediated procedures produce the desired compounds in high yields and are operationally simple but also exhibit drawbacks such as cost and environmentally friendliness. With the limited number of systems reported towards the TOP-mediated Wittig reaction we believe that this area has huge untapped potential and that more research is certainly required.
5. Synthesis of imines
The synthesis of imines has been reported using a MnO2 mediated TOP, however, owing to the vast importance of imines in synthetic organic chemistry,34 the one pot mediated imine synthesis using a variety of oxidants continues to garner widespread interest. The imine moiety is particularly valued in chemistry as it is used in synthetic organic chemistry and pharmaceutical and agricultural chemical production.35,365.1 K-OMS-2
Having previously mentioned the use manganese octrahedral sieves (K-OMS-2) to effect the synthesis of quinoxalines it is probably unsurprising to find that the same research group has used K-OMS-2 to synthesize imines via a one pot tandem oxidation process (Scheme 20).37 Using this approach, a series of imines were formed in excellent yields using aromatic alcohols however, the system was less effective on aliphatic alcohols. K-OMS-2 was shown to play a role in the oxidation and the coupling process, as inferior yields were obtained when attempting the coupling of aldehydes and amines in the absence of OMS-2.5.2 Palladium
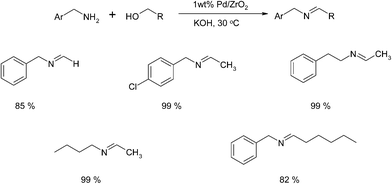
As can be seen, the synthesis of imines from aliphatic alcohols remained a great challenge (vide infra). Thus, the development of a Pd/ZrO2 system was a welcome addition as special emphasis was placed on developing the coupling between aliphatic alcohols and amines in which the alcohol served as reactant and solvent.38 Using this approach, a series of aliphatic and aromatic amines were coupled with a variety of aliphatic alcohols. A selection of the results obtained is highlighted below (Scheme 21).Interestingly, the carbonyl intermediate was not detected by GC-MS and it is therefore believed that the oxidation is the rate determining step and the aldehyde is produced in low concentration and immediately trapped by the nucleophile to produce the imine.
A solvent-free, low catalyst loading of Pd has also been reported for the synthesis of imines. This Pd(OAc)2/NEt3/TEMPO/t-BuOK system is advantageous from an environmental perspective as it is conducted in the absence of solvent and requires low Pd loading (1 mol%) (Scheme 22).39
At this point, it perhaps appropriate to mention an interesting trend that is developing in the area of tandem oxidation process coupling reactions. The two most common pathways to access this assortment of valuable compounds from alcohols, is the dehydrogenation pathway and the traditional alcohol oxidation with both procedures involving the formation of the aldehyde which is subsequently elaborated further. However, certain groups have chosen to clearly differentiate between the two pathways as the dehydrogenation pathway produces H2 gas as a by-product. However, for the purposes of this review, we have chosen to include the dehydrogenation pathway as it provides valuable synthetic routes to a variety of compounds commencing from the alcohol. Our decision was further supported by two review articles by the Crabtree research group and the Williams research group in which they commented on the merits of a TOP through the dehydrogenation pathway. Crabtree and co-workers while compiling a review on ‘Dehydrogenation as a Substrate-Activating Strategy in Homogenous Transition-Metal Catalysis’ noted that the alcohol can be converted to a carbonyl compound, which reacts further in “tandem ‘one pot’ procedure”.40 The Williams' group review on ‘Metal-catalyzed approaches to Amide bond formation’ commented that the formation of the amide from the alcohol in which hydrogen being the only by-product is an “exceptionally clean and simple reaction”. They further elaborate by noting that “the general mechanism is the same as that of oxidative amination involving aldehydes and amines, but with an additional oxidation step at the start to convert the alcohol to the aldehyde”.41 Based on these comments, we felt it would be remiss of us not to include the dehydrogenation pathway mediated coupling reactions.
The direct synthesis of imines from assorted alcohols and amines was also achieved using a Pd/DNA catalyst in an aqueous solvent.42 These Pd/DNA hybrids were prepared by mixing PdCl2 and fish sperm DNA.43 The Pd/DNA particles provided imines in excellent yields with the liberation of H2. By this approach, a series of alcohols and amines were coupled to produce the desired imines in high yields (Scheme 23).
A recycling study of the Pd/DNA complex revealed that a minor loss in activity was evident after the fifth recycling. The authors then proceeded to develop a TOP imine synthesis commencing from the alcohol and nitroarene 18 in which the nitroarene is deoxidized into the amine and subsequent condensation of the aldehyde and the generated amine produces the desired imine (Scheme 24).
5.3 Gold
The synthesis of imines has also been carried out using a polymer incarcerated gold/palladium alloy nanoparticles with molecular oxygen as an oxidant.44 The system was generalized to a wide variety of substrates with excellent activity observed (Scheme 25). This combination of Au and Pd generates a bimetallic alloy structure which displays different properties to the individual metals as the Pd expresses more electropositive properties while Au displays more electronegative properties.The use of supported gold has also emerged as a viable catalyst for TOP mediated imine synthesis. Hensen and co-workers have reported a basic hydrotalcite (HT)-supported gold nanoparticle promoted imine synthesis under mild and soluble-base-free conditions.45 Hydrotalcite (HT: Mg6Al2(OH)16CO3·nH2O) type layered double hydroxides possess surface basicity which can be fine-tuned by their composition. Their performance can be adjusted by varying the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg ratio. Various alcohols and amines were evaluated using the (HT)-supported gold nanoparticle system and the desired imines were formed in excellent yields as demonstrated in the following table (Table 4).
Mg ratio. Various alcohols and amines were evaluated using the (HT)-supported gold nanoparticle system and the desired imines were formed in excellent yields as demonstrated in the following table (Table 4).
Gold nanoparticles on TiO2 in combination with potassium methoxide have also been used as an oxidant in TOP mediated imine synthesis.46 While this particular system was selectively formed the imine the conversion was relatively disappointing as illustrated below (Scheme 26). (Selectivity in parentheses).
5.4 Platinum
TiO2 loading Pt particles (Pt@TiO2) have also been shown to promote the one pot synthesis of imines from alcohols and amines under UV irradiation.47 This procedure was envisioned to occur by firstly, the oxidation of the alcohol to the aldehyde via Pt-assisted photocatalytic oxidation on the TiO2 surface and secondly, the condensation of the amine and aldehyde on the Lewis acid site on the TiO2 surface. The performance of Pt@TiO2 was compared with pure TiO2 and the titanium loaded particles were found to be far superior to pure TiO2 (Table 5).5.5 Copper
The use of a copper catalyzed TOP imine synthesis using oxygen as a green oxidant has also been reported with great success.48 This procedure, reported by Zhang and co-worker evaluated the application of a copper catalyst in the synthesis of imines. Of the catalysts evaluated, it was found that Cu(ClO4)2·6H2O in combination with KOH under an oxygen atmosphere effectively promotes the formation of imines (Table 6). The procedure was effective using benzyl alcohol and a wide variety of amines however, when an aliphatic or secondary alcohol was used the designed system was less effective as illustrated below.At the conclusion of this study, the authors' monitored the effect of decreasing the catalyst loading on the yield. It was found that as little as 0.005 mol% of Cu(ClO4)2·6H2O could still catalyze the coupling of benzyl alcohol and benzyl amine in 82% yield in 72 hours.
5.6 Iron
Iron has also been used to catalyze a TOP assisted imine synthesis via an Fe(NO3)3/TEMPO system.49 As with the use of copper, the iron mediated system required the presence of base to proceed efficiently and the addition of KOH was found to be essential for a successful coupling reaction. Using this approach, the coupling reactions were particularly successful when aromatic amines and aromatic alcohols were used. However, when aliphatic amines or aliphatic alcohols were used disappointing yields or no products were detected as illustrated below (Table 7).5.7 Ruthenium
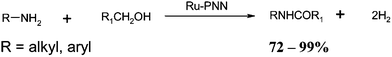
The final transition metal mediated TOP imine synthesis to be discussed is the use of ruthenium complexes. This procedure involves mixing an alcohol, amine, ruthenium complex in toluene under refluxing conditions. There are two complexes, synthesized by David Milstein's group that has been used successfully for the TOP imine synthesis. The complexes illustrated below have been designated as “Milstein's PNP” and “Milstein's PNN” complexes (Fig. 3).In 2010, the Milstein group reported the synthesis of imines via one pot TOP using the PNP complex.50 This reaction leads to the liberation of hydrogen gas and water and no waste products (Scheme 27). Remarkably, the synthesized imine does not undergo hydrogenation under the present experimental conditions.
While in 2012, the Schomaker group evaluated the PNN complex with comparable success.51 The synthesis of α, β-unsaturated imines from primary allylic alcohols and amines is certainly noteworthy (Scheme 28).
5.8 Iridium
Peris and co-workers reported an interesting approach, by the use of a nitroarene in the imine synthesis, using a range of iridium–palladium complexes (Scheme 29).52 This procedure involves (i) the reduction of the nitroarene to amine, (ii) the oxidation of the alcohol to the aldehyde and (iii) condensation of the aldehyde and the amine to form the corresponding imine.While the use of transition metal mediated TOP has been well documented, there are other interesting reports. In some of the procedures highlighted above, the transition metal catalyst is used in combination with a strong base. Recently, (an almost simultaneously) the Adimurthy research group and the Tang research group reported the synthesis of imines using an aerobic oxidation using NaOH53 and KOH54 respectively.
The Adimurthy group reported that just 10 mol% of NaOH can catalyze the coupling of benzyl alcohol and benzylamine to produce the imine under solvent free conditions under open atmosphere in 20 hours (Scheme 30).
It may be possible that trace amounts of transition metals, in commercially available NaOH, is catalyzing the above coupling reaction. To disprove this theory, the authors' carried out the coupling reaction using pure Na metal and NaH and yields similar to those obtained with NaOH were obtained. Finally, to confirm these findings the reaction was conducted with preformed sodium alkoxide of benzyl alcohol and a 98% yield and selectivity was observed, indicating that a sodium alkoxide species is formed as part of an intermediate. These observations also decrease the likelihood of trace transition metals playing a role in the catalytic system.
The Tang research group obtained similar results to the Adimurthy research group, however using KOH in refluxing toluene in ambient conditions, and used a comparable rationale to discount the influence of trace transition metals on the catalytic system.
The determination of the ideal system for TOP-mediated imine system is a complex one as all the procedures discussed have a number of positive and negative attributes. The simplest systems involve the use copper and iron catalysts but these are limited applicability on aliphatic alcohols. The use of strong bases to catalyze the synthesis of imines is certainly interesting and was indeed successful on the TOP-mediated imine system but, given the corrosive nature of the environment, the application of these reaction conditions to other TOP systems will be difficult. Thus, in our opinion, the Pd/ZrO2 is the best option as it uses low catalyst loadings (1 wt%), is conducted an atmospheric conditions, produces the imines in high yields and perhaps most importantly is applicable to a wide range of substrates including aliphatic alcohols. This particular area of TOP is perhaps, the most popular and a number of interesting research approaches being reported including a particularly remarkable one pot procedure for the formation of alcohols from the imine!55
6. Synthesis of amides
The amide bond is one of the most valuable moieties in synthetic organic chemistry56 and therefore has been synthesized using TOP.6.1 Ruthenium
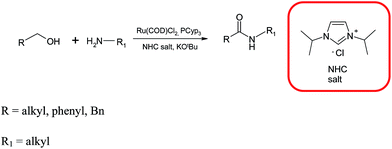
The landmark publication in this research was by the Milstein research group in which they described the coupling between an alcohol and amine using a ruthenium pincer complex with molecular hydrogen being the only by-product (Scheme 31).57 Using this approach, the alcohol is oxidized to the aldehyde 19 and further oxidized to the hemiaminal 20 to produce the amide (Scheme 32). This report spawned a series of amide syntheses, commencing from the alcohol and the most pertinent of which, will be discussed below.Hong and co-workers have also developed a procedure for the direct synthesis of amides from alcohols and amines using a RuCl3, a N-heterocyclic carbene (NHC), sodium hydride and pyridine system (Scheme 33).58
A similar system with comparable yields was also reported using a ruthenium precursor, an N-heterocyclic carbine and a phosphine ligand (Scheme 34).59,60
6.2 Gold/cobalt
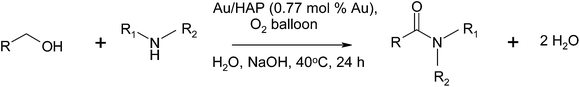
Kobayashi and co-workers reported the synthesis of amides using an alcohol and amine in which heterogeneous gold and gold/cobalt nanoparticles were used as catalysts with oxygen as the terminal oxidant (Scheme 35).61 They make special attention to differentiate their process from the dehydrogenative pathway as the only by-product in this process is water. The use of either polymer-incarcerated carbon black (PICB) either with gold or gold/cobalt nanoparticles shows excellent activity depending on the type of alcohols used. The PICB–Au catalyst was effective on nonbenzylic alcohols while PICB–Au/Co was effective on benzylic alcohols.Wang and co-workers described the synthesis of amides from alcohols and amines using a hydroxyapatite-supported gold catalyst (Au-HAP) with up to 99% yield.62 The reaction was conducted in water, which is particularly attractive from an environmental aspect, and was effective on various aromatic and aliphatic amines and alcohols (Table 8).
6.3 Aqueous ammonia and OMS-2
Amides were also directly synthesized from primary alcohols and aqueous ammonia and O2 in the presence of manganese oxide based octahedral sieves (KMn8O16; OMS-2) which have a 2 × 2 hollandite structure with a one-dimensional pore.63,64 The system was particularly effective on benzylic alcohols although a decrease in yield was observed when aliphatic alcohols were tested (Scheme 36).The variety routes discussed to access the amide moiety use a variety of complex reagents which nevertheless provide the desired compounds in high yields. The manganese dioxide octahedral sieve method is certainly attractive due to its simplicity however a decrease in yield in observed when aliphatic alcohols are tested. The PICB with gold and cobalt is applicable to a wide range of substrates, produces the desired amide in good to excellent using mild reaction conditions and while the catalysts are recoverable and reusable the catalyst preparation time of 72 h is a disadvantage.
7. Synthesis of nitriles
The nitrile moiety is a valuable group in synthetic organic chemistry as it is present in a number of fine chemicals, pharmaceuticals and dyes.65 Consequently, the synthesis of nitriles from alcohols via the tandem oxidation process has received much attention.7.1 Stoichiometric I2/DIH
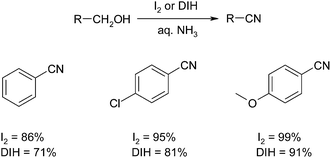
The synthesis of nitriles was achieved from alcohols by comparing the performance of molecular iodine (I2) and 1,3-diiodo-5,5-dimethylhydantoin (DIH) as stoichiometric oxidants.66 Both molecular iodine and DIH were found to effectively promote the synthesis of nitriles with excellent yields reported in both cases as illustrated below (Scheme 37).The use of iodine as a catalyst was further explored with the report of a comparison of KI or I2 in combination with tert-butyl hydroperoxide (TBHP) and aqueous NH3 for the one-pot conversion of alcohols to nitriles.67 The use of KI or I2 as the catalyst provided the desired nitrile in comparative yields (Scheme 38).
7.2 Ruthenium
The direct conversion of alcohols to nitriles was extended from the use of stoichiometric oxidants to catalytic oxidants. The catalytic oxidative synthesis was reported by Mizuno and co-workers from primary alcohols and ammonia using a ruthenium hydroxide catalyst with oxygen (or air) as the sole oxidant.68 Using this approach, a series of nitriles were prepared in excellent yields. This procedure is most appealing as it avoids the use of stoichiometric amounts of reactive oxidants and reagents as previously described. The ruthenium catalyzed TOP was effective on benzylic, allylic and heteroatom-containing alcohols as illustrated below (Scheme 39).7.3 Copper
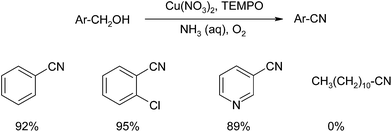
Tao and co-workers have reported the direct conversion of benzylic alcohols into nitriles using a copper catalyst, NH3 as the nitrogen source, O2 as the oxidant and 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO) (Scheme 40).69 The system was effective in the synthesis of nitriles using a range of benzylic alcohols and excellent yields were obtained. When the system was applied to aliphatic alcohols, no product was obtained which may be due to the inherent limitations of TEMPO on these substrates70,71 although the authors make no mention of this.Of the systems discussed for a TOP-mediated nitrile system, the I2/DIH system is, in our opinion, the most attractive as it:
• is applicable to a wide range of substrates
• produces the desired nitriles in excellent yields
• is operationally simple.
8. New additions to TOP
The interesting systems described above were elegantly reported by the Taylor group using MnO2 as an oxidant. In recent times, new research groups have been extending the ground-breaking work of the Taylor research group and this has been mainly achieved by the introduction of novel oxidants to the TOP domain. However, an alternate approach is the application of TOP to interesting and undiscovered systems and one such system is a multicomponent tandem oxidation process.8.1 Multicomponent reactions – Passerini reaction
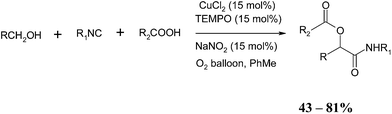
In 2006, Zhu and co-workers reported the synthesis of multicomponent TOP in a Passerini reaction. The Passerini reaction, first reported in 1921 by Italian chemist Mario Passerini, produces an α-acyloxy carboxamide from an aldehyde, isonitrile and carboxylic acid.72,73 Zhu and co-workers reported that this reaction can be successfully achieved commencing from the alcohol 21, isonitrile 22 and carboxylic acid 23 using IBX as an oxidant to produce the desired α-acyloxy carboxamide 24 (Scheme 41).74The optimization study revealed that THF was the ideal solvent and these conditions were applied to a range of alcohols, isonitriles and carboxylic acids and a selection of the products obtained is highlighted below. The glucofuranose derivative 25 is especially significant as the corresponding aldehyde was shown, by the authors', to be unstable (Scheme 42).
The use of excess IBX remained a drawback of the above methodology and in subsequent publications the authors' report a three-component Passerini reaction commencing from alcohols under catalytic conditions using oxygen as the terminal oxidant.75 Using this approach, the desired α-acyloxy carboxamides were formed in good yields as shown in the following scheme (Scheme 43).
8.2 Multicomponent reactions – imidazole synthesis
Shaabani and co-workers reported the three component synthesis of trisubstituted imidazoles using a ceric ammonium nitrate (CAN) catalyzed aerobic oxidation of α-hydroxy ketones (Scheme 44).76 Using this approach, a series of 2,4,5-trisubstituted imidazole derivatives were synthesized in yields up to 85% using α-hydroxy ketones, aldehydes and ammonium acetate in the presence of a catalytic amount of CAN under aerobic oxidation conditions.8.3 Synthesis of 4H-chromene derivatives
Rueping and co-workers have reported the synthesis of 4H-chromene derivatives using a TOP approach.77 This elegant synthesis was envisioned to occur by merging a catalytic oxidative process in situ for the formation of the aldehyde with a secondary amine – catalyzed enantioselective cascade (Scheme 45).Using tetrapropylammonium perruthenate, TPAP with N-methylmorpholine-N-oxide (NMO) as the catalytic oxidative system, the synthesis of 4H-chromene derivatives were formed in excellent yields and enantioselectivites (Table 9).
8.4 Synthesis of esters
A tandem oxidation approach towards the synthesis of esters from allylic and benzylic alcohols catalyzed by N-heterocyclic carbenes and MnO2 has also been reported.78 A brief optimization study was conducted and a simple triazolium salt was found to efficiently promote the synthesis of esters. With this optimized procedure in hand, the system was explored using a range of substrates. As can be seen from the data below, the system was effective on allylic, propargyl and heteroaromatic substrates (Scheme 46).9. Total syntheses using a TOP approach
The pillars of TOP such as decrease in time, higher yields and fewer synthetic steps are valuable in total synthesis. The improved yields, in particular, are attractive as many successful total syntheses report low yields of the final product. Thus, the use of TOP in total synthesis is useful when problematic intermediates are encountered and as an approach to improve the final yield of the product.9.1 Synthesis of Tedanalactam
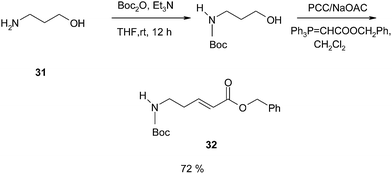
Tedanalactam, a cis-3,4-epoxy-2-piperidone 26, was first isolated from the sponge Tedania ignis by Cronan79 and co-worker in 1994 and has been found to display promising fungicidal activity. In 2009, Tilve and co-workers reported the total synthesis of Tedanalactam using the TOP mediated Wittig reaction as a crucial step.80 The retrosynthesis of Tedanalactam is outlined below (Scheme 47) which originates from 3-tosyloxy-4-hydroxy-2-piperidone 27 which is assembled from the δ-amino-ester 28. This monotosyl ester can be prepared from the corresponding dihydroxy ester 29 to be obtained by Sharpless asymmetric dihydroxylation of the unsaturated ester 30.The unsaturated ester is therefore a key intermediate en route to Tedanalactam and was prepared using the tandem oxidation process. The reaction commences with 3-amino-1-propanol 31 which is subjected to N-protection using Boc anhydride and subsequently transformed into the olefin 32, in 72% yield, using the tandem oxidation process using a PCC/NaOAc system (Scheme 48).
9.2 Synthesis of (±)-cuevaene A
In 2012, Taylor and co-worker reported the total synthesis and structural confirmation of (±)-cuevaene A 33.81 The retrosynthesis proposed by the authors' consisted of generating the key aldehyde which could be accessed by the homologation of the ketone which in turn is accessed from cyclohexa-1,3-dione and quinone monoketal (Scheme 49).The synthetic route commenced by dearomatizing quinone monketal 37 from 4-methoxyphenol 38 using phenyliodonium trifluoroacetate (PIFA). This was followed by a tBuOK-catalyzed double conjugate addition, followed by an acidic rearrangement using hydrochloric acid. The obtained ketone was subjected methylenation, protection, hydroboration and oxidations producing the desired aldehyde 39 in 39% yield from the phenol derivative (Scheme 50).
The obtained aldehyde 39 was converted to the ester 40 which was subsequently reduced using DIBAL-H to produce the alcohol 41. The alcohol was oxidized using MnO2 to the aldehyde 42 and subjected to a Wadsworth–Emmons reaction to produce the diene 43. The diene was reduced using LiBH4 to produce the alcohol 44. The obtained alcohol was unstable in a solution of chloroform and the final alkene 45 was obtained via a TOP mediated approach. Finally, a deprotection and hydrolysis was carried out to produce cuevaene A 33 in a yield of 7% over 14 steps (Scheme 51).
10. Conclusions and outlook
The tandem oxidation process has developed into a valuable addition to synthetic organic chemistry. Since the early days of TOP with the Huang group to the massive contribution of the Taylor group, TOP continues to find widespread interest to chemists the world over. In recent times, a number of groups have reported their research efforts into TOP with the use of novel and environmentally friendly oxidants. The use of oxygen, in particular, as the terminal oxidant is always attractive from an environmental point of view. Additionally, extending the TOP to multicomponent systems has resulted in a number of new synthetically useful compounds. While, at first glance, it appears that the possibilities for a TOP is endless, the situation is rather complex. The challenge lies in assembling a system in which the alcohol, nucleophile, oxidant, additives and even the solvent can co-exist. Numerous examples exist in which the oxidation of the alcohol to aldehyde is successful however no coupling product is detected using the TOP. A possible solution may be to develop simple, high yielding alcohol oxidation procedures before application to the TOP. The simpler the alcohol oxidation system the greater chance of a successful coupling reaction as there is fewer reagents to derail the coupling process. The last nine years have certainly been exciting for the TOP and given the continued interest in this research, the future is indeed bright.Abbreviations
| BaMnO4 | Barium manganate |
| Bn | Benzyl |
| Ph3PCHCO2CH2Bn | Benzyl(triphenylphosphoranylidene)acetate |
| Cu(ClO4)·6H2O | Copper(II) perchlorate hexahydrate |
| COD | Cyclooctadiene |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| Me2SO | Dimethyl sulfoxide |
| DIBAL-H | Diisobutylaliminium hydride |
| Boc2O | Di-tert-butyldicarbonate |
| Ph3PCH(Me)CO2Et | Ethyl-2-triphenylphoranyldenepropionate |
| Ga(ClO4)3 | Gallium(III) perchlorate |
| IBX | 2-Iodooxybenzoic acid |
| LiBH4 | Lithium borohydride |
| LiOH·H2O | Lithium hydroxide monohydrate |
| Ph3PCHCO2Me | Methyl(triphenylphosphoranyldene)acetate |
| MPA | 12-Molybdophosphoric acid |
| (COCl)2 | Oxalyl chloride |
| Pd(OAc)2 | Palladium(II) acetate |
| KHMDS | Potassium bis(trimethylsilyl)amide |
| K2CO3 | Potassium carbonate |
| t-BuOK | Potassium tert-butoxide |
| KOCH3 | Potassium methoxide |
| RuCl2(PPh3)3 | Ruthenium(II) dichlorotris(trisphenylphosphine) |
| NaNO2 | Sodium nitrite |
| Boc | tert-Butyloxycarbonyl |
| TBDMS | tert-Butyldimethylsilyl ether |
| TEMPO | 2,2,6,6-Tetramethylpiperidine-1-oxyl radical |
| PCyp3 | Tricyclopentylphosphine |
| TEA | Triethylamine |
| Ph3PCHCHO | (Triphenylphosphoranylidene)acetaldehyde |
| Ts | Tosyl |
| ZrO2 | Zirconium dioxide |
References
- L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed., 1993, 105, 137–170 CAS.
- L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed.
- R. E. Ireland and D. W. Norbeck, J. Org. Chem., 1985, 50, 2198–2200 CrossRef CAS.
- C. C. Huang, J. Labelled Compd. Radiopharm., 1987, 24, 676–681 CrossRef PubMed.
- R. J. K. Taylor, L. Alcaraz, I. Kapfer-Eyer, G. Macdonald, X. Wei and N. J. Lewis, Synthesis, 1998, 775–790 CrossRef CAS PubMed.
- G. Macdonald, L. Alcaraz, X. Wei, N. J. Lewis and R. J. K. Taylor, Tetrahedron, 1998, 54, 9823–9836 CrossRef CAS.
- L. Alcaraz, G. Macdoanld, J. Ragot, N. J. Lewis and R. J. K. Taylor, J. Org. Chem., 1998, 63, 3526–3527 CrossRef CAS.
- L. Alcaraz, G. Macdoanld, J. Ragot, N. J. Lewis and R. J. K. Taylor, Tetrahedron, 1999, 55, 3707–3716 CrossRef CAS.
- A. G. M. Barrett, D. Hamprecht and M. Ohkubo, J. Org. Chem., 1997, 62, 9376–9378 CrossRef CAS.
- S. Shuto, S. Niizuma and A. Matsuda, J. Org. Chem., 1998, 63, 4489–4493 CrossRef CAS.
- D. Crich and X.-S. Mo, Synlett, 1999, 67–68 CrossRef CAS PubMed.
- R. J. K. Taylor, M. Reid, J. Foot and S. A. Raw, Acc. Chem. Res., 2005, 38, 851–869 CrossRef CAS PubMed.
- E. Quesada, S. A. Raw, M. Reid, E. Roman and R. J. K. Taylor, Tetrahedron, 2006, 62, 6673–6680 CrossRef CAS PubMed.
- Y. F. Shen, R. P. Zerger, R. N. DeGuzman, S. L. Suib, L. McCurdy, D. I. Potter and C. L. O'Young, Science, 1993, 260, 511–515 CAS.
- S. Sithambaram, Y. Ding, W. Li, X. Shen, F. Gaenzler and S. L. Suib, Green Chem., 2008, 10, 1029–1032 RSC.
- J. Sharma, S. Nahima, B. A. Kakade, R. Pasricha, A. B. Mandale and K. Vijayamohanan, J. Phys. Chem., 2004, 108, 13280–13286 CrossRef CAS.
- T. Bhattacharya, T. K. Sarma and S. Samanta, Catal. Sci. Technol., 2012, 2, 2216–2220 CAS.
- S. C. Cho and G. O. Sung, J. Mol. Catal. A: Chem., 2007, 276, 205–210 CrossRef PubMed.
- C. S. Cho and W. X. Ren, J. Organomet. Chem., 2009, 694, 3215–3217 CrossRef CAS PubMed.
- S. C. Cho and G. O. Sung, Tetrahedron Lett., 2006, 2006, 5633–5636 CrossRef PubMed.
- K. T. Venkateswara Rao, P. S. Sai Prasad and N. Lingaiah, J. Mol. Catal. A: Chem., 2009, 312, 65–69 CrossRef CAS PubMed.
- W. Song, P. Liu, M. Lei, H. You, X. Chen, H. Chen, L. Ma and L. Hu, Synth. Commun., 2012, 42, 236–245 CrossRef CAS.
- F. Pan, T.-M. Chen, J.-J. Cao, J.-P. Zou and W. Wang, Tetrahedron Lett., 2012, 53, 2508–2510 CrossRef CAS PubMed.
- V. Jeena and R. S. Robinson, Beilstein J. Org. Chem., 2009, 5, 24 Search PubMed.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS PubMed.
- S. E. Wolkenberg, D. D. Wisnoski, W. H. Leister, Y. Wang, Z. Zhao and C. W. Lindsley, Org. Lett., 2004, 6, 1453–1456 CrossRef CAS PubMed.
- S. Y. Kim, K. H. Park and Y. K. Chung, Chem. Commun., 2005, 1321–1323 RSC.
- V. Jeena and R. S. Robinson, Tetrahedron Lett., 2014, 55, 642–645 CrossRef CAS PubMed.
- P. Ghosh, A. Mandal and R. Subba, Catal. Commun., 2013, 41, 146–152 CrossRef CAS PubMed.
- A. S. Kireev, O. N. Nadein, V. J. Agustin, N. E. Bush, A. Evidente, M. Manpadi, S. K. Ogasawara, S. K. Rastogi, S. Rogelj, S. T. Shors and A. Kornienko, J. Org. Chem., 2006, 71, 5694–5707 CrossRef CAS PubMed.
- S. Lang and R. J. K. Taylor, Tetrahedron Lett., 2006, 47, 5489–5492 CrossRef CAS PubMed.
- E. Y. Lee, Y. Kim, J. S. Lee and J. Park, Eur. J. Org. Chem., 2009, 2943–2946 CrossRef CAS PubMed.
- G. H. Harris and A. E. Graham, Tetrahedron Lett., 2010, 52, 6890–6892 CrossRef PubMed.
- J.-H. Xie, S.-F. Zhu and Q.-L. Zhou, Chem. Rev., 2011, 111, 1713–1760 CrossRef CAS PubMed.
- W. Tang and X. Zhang, Chem. Rev, 2003, 103, 3029–3069 CrossRef CAS PubMed.
- J. P. Adams, J. Chem. Soc., Perkin Trans. 1, 2000, 125–139 RSC.
- S. Sithambaram, R. Kumar, Y.-C. Son and S. L. Suib, J. Catal., 2008, 253, 269–277 CrossRef CAS PubMed.
- W. Cui, B. Zhaorigetu, M. Jia, W. Ao and H. Zhu, RSC Adv., 2014, 4, 2601–2604 RSC.
- L. Jiang, L. Jin, H. Tian, X. Yuan, X. Yu and Q. Xu, Chem. Commun., 2011, 47, 10833–10835 RSC.
- G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681–703 CrossRef CAS PubMed.
- C. L. Allen and J. M. J. Williams, Chem. Soc. Rev., 2011, 40, 3405–3415 RSC.
- L. Tang, H. Sun, Y. Li, Z. Zha and Z. Wang, Green Chem., 2012, 14, 3423–3428 RSC.
- Y. Wang, G. H. Ouyang, J. T. Zhang and Z. Y. Wang, Chem. Commun., 2010, 46, 7912–7914 RSC.
- J.-F. Soule, H. Miyamura and S. Kobayashi, Chem. Commun., 2013, 49, 355–357 RSC.
- P. Liu, C. Li and E. J. M. Hensen, Chem.–Eur. J., 2012, 18, 12122–12129 CrossRef CAS PubMed.
- S. Kegnes, J. Mielby, U. V. Mentzel, C. H. Christensen and A. Rissager, Green Chem., 2010, 12, 1437–1441 RSC.
- Y. Shiraishi, M. Ikeda, D. Tsukamoto, S. Tanaka and T. Hirai, Chem. Commun., 2011, 47, 4811–4813 RSC.
- Q. Kang and Y. Zhang, Green Chem., 2012, 14, 1016–1019 RSC.
- E. Zhang, H. Tian, S. Xu, X. Yu and Q. Xu, Org. Lett., 2013, 15, 2704–2707 CrossRef CAS PubMed.
- B. Gnanaprakasam, J. Zhang and D. Milstein, Angew. Chem., Int. Ed., 2010, 49, 1468–1471 CrossRef CAS PubMed.
- J. W. Rigoli, S. A. Moyer, S. D. Pearce and J. M. Schomaker, Org. Biomol. Chem., 2012, 10, 1746–1749 CAS.
- A. Zanardi, J. A. Mata and E. Peris, Chem.–Eur. J., 2010, 16, 10502–10506 CrossRef CAS PubMed.
- R. R. Donthiri, R. D. Patil and S. Adimurthy, Eur. J. Org. Chem., 2012, 4457–4460 CrossRef CAS PubMed.
- J. Xu, R. Zhuang, L. Bao, G. Tang and Y. Zhao, Green Chem., 2012, 14, 2384–2387 RSC.
- C. Solé, A. Whiting, H. Gulyás and E. Fernández, Adv. Synth. Catal., 2011, 353, 376–384 CrossRef PubMed.
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 CAS.
- C. Gunanathan, Y. Ben-David and D. Milstein, Science, 2007, 317, 790–792 CrossRef CAS PubMed.
- S. C. Ghosh and S. H. Hong, Eur. J. Org. Chem., 2010, 4266–4270 CrossRef CAS PubMed.
- L. U. Nordstrom, H. Vogt and R. Madsen, J. Am. Chem. Soc., 2008, 130, 17672–17673 CrossRef CAS PubMed.
- J. H. Dam, G. Osztrovsky, L. U. Nordstrom and R. Madsen, Chem.–Eur. J., 2010, 16, 6820–6827 CrossRef CAS PubMed.
- J.-F. Soule, H. Miyamura and S. Kobayashi, J. Am. Chem. Soc., 2011, 133, 18550–18553 CrossRef CAS PubMed.
- W. Wang, Y. Cong, L. Zhang, Y. Huang, X. Wang and T. Zhang, Tetrahedron Lett., 2104, 55, 124–127 CrossRef PubMed.
- K. Yamaguchi, H. Kobayashi, T. Oishi and N. Mizuno, Angew. Chem., Int. Ed., 2012, 51, 544–547 CrossRef CAS PubMed.
- K. Yamaguchi, H. Kobayashi, Y. Wang, T. Oishi, Y. Ogasawara and N. Mizuno, Catal. Sci. Technol., 2013, 3, 318–327 CAS.
- F. F. Fleming, L. Yao, P. C. Ravikumar, L. Funk and B. C. Shook, J. Med. Chem., 2010, 53, 7902–7917 CrossRef CAS PubMed.
- S. Iida and H. Togo, Tetrahedron, 2007, 63, 8274–8281 CrossRef CAS PubMed.
- K. R. Reddy, C. U. Maheswari, M. Venkateshwar, S. Prashanthi and M. L. Kantam, Tetrahedron Lett., 2009, 50, 2050–2053 CrossRef PubMed.
- T. Oishi, K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2009, 121, 6404–6406 CrossRef PubMed.
- C. Tao, F. Liu, Y. Zhu, W. Liu and Z. Cao, Org. Biomol. Chem., 2013, 11, 3349–3354 CAS.
- S. S. Kim and H. C. Jung, Synthesis, 2003, 2135–2137 CrossRef CAS.
- V. Jeena and R. S. Robinson, Chem. Commun., 2012, 48, 299–301 RSC.
- M. Passerini, Gazz. Chim. Ital., 1921, 51, 126–129 CAS.
- M. Passerini, Gazz. Chim. Ital., 1922, 52, 432–435 CAS.
- T. Ngouansavanh and J. Zhu, Angew. Chem., Int. Ed., 2006, 45, 3495–3497 CrossRef CAS PubMed.
- J. Brioche, G. Masson and J. Zhu, Org. Lett., 2010, 12, 1432–1435 CrossRef CAS PubMed.
- A. Shaabani, A. Maleki and M. Behnam, Synth. Commun., 2009, 39, 102–110 CrossRef CAS.
- M. Rueping, J. Dufour and M. S. Maji, Chem. Commun., 2012, 48, 3406–3408 RSC.
- B. E. Maki, A. Chan, E. M. Phillips and K. A. Scheidt, Org. Lett., 2007, 9, 371–374 CrossRef CAS PubMed.
- J. M. J. Cronan and J. H. I. Cardellina, Nat. Prod. Lett., 1994, 5, 85–88 CrossRef CAS.
- M. S. Majik, P. S. Parameswaran and S. G. Tilve, J. Org. Chem., 2009, 74, 6378–6381 CrossRef CAS PubMed.
- P. G. E. Craven and R. J. K. Taylor, Tetrahedron Lett., 2012, 53, 5422–5425 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |