A highly efficient Nafion-H catalyst for vapour phase carbonylation of dimethoxymethane†
Shiping Liuab,
Wenliang Zhua,
Lei Shia,
Hongchao Liua,
Yong Liua,
Youming Nia,
Lina Liab,
Hui Zhouab,
Shutao Xua and
Zhongmin Liu*a
aNational Engineering Laboratory for Methanol to Olefins, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: liuzm@dicp.ac.cn; Fax: +86-411-84691570; Tel: +86-411-84685510
bUniversity of Chinese Academy of Sciences, Beijing, 100049, P. R. China
First published on 12th August 2014
Abstract
A highly active Nafion-H catalyst is developed for vapour phase carbonylation of dimethoxymethane (DMM) to methyl methoxyacetate (MMAc) with a significant MMAc selectivity as high as 90%. The excellently catalytic performance is because of the unique structure and high acid strength of the Nafion-H catalyst.
Ethylene glycol (EG), which is widely used as automotive antifreeze and an intermediate for polyester resins and fibers,1 had a global production capacity of more than 23 Mtons in 2012.2 Currently, EG is mainly produced from ethylene.3 However, the rapid depletion of petroleum reserves leads to the gradual rise in ethylene prices, which has driven to the consideration of alternative starting materials. Syngas, which is a mixture of CO and H2 can be generated by reforming of natural gas, gasification of coal and renewable biomass, is a potential candidate for the synthesis of EG through the carbonylation of formaldehyde to glycolic acid, followed by esterification and hydrogenation to EG.4–10
Typically, carbonylation of formaldehyde is exclusively conducted in liquid phase with strong liquid mineral acids as homogenous catalysts.7,8 However, the usage of strong liquid mineral acids results in serious corrosion problems. Furthermore, the solubility of CO in most of the solvents is poor; therefore, higher pressures are required to promote the concentration of CO in liquid phase. According to our acknowledge, no literature studies have reported the carbonylation of formaldehyde in a single vapour phase because formaldehyde can be readily polymerized to polyformaldehyde under mild conditions.11 For the first time, Bell and co-workers reported the vapour phase carbonylation of dimethoxymethane (DMM) using H-Faujasite (H-FAU, an acid zeolite) as the catalyst to synthesize methyl methoxyacetate (MMAc) with a selectivity of up to 79% and a yield of up to 20%.12 However, their further study showed that the steric constraint of the framework of zeolites could promote the disproportionation of DMM, which led to decrease in the selectivity for MMAc.13 In this work, we report a highly active catalyst (Nafion-H resins), which also exhibits considerably higher selectivity for the carbonylation of DMM to MMAc. The excellent catalytic performance of Nafion-H resins is attributed to their unique structure and chemical properties.
Fig. 1 shows the effect of reaction temperatures on the conversion of DMM and products distribution in the range from 80 to 150 °C. At 80 °C, the conversion of DMM is low, about 15%. However, the Nafion-H catalyst presents a surprisingly high selectivity for MMAc (nearly 90%). In addition to the main product, other by-products including dimethyl ether (DME), methyl formate (MF) and methanol are observed. With gradually increasing the reaction temperature to 110 °C, the conversion of DMM significantly increases from about 15 to 54% and the selectivity for MMAc only slightly decreases. Further increasing the reaction temperature only leads to a slight increase in the DMM conversion, but the selectivity for MMAc sharply decreases to 70% at 150 °C because DMM favors the disproportionation more than the carbonylation reaction at higher temperature. Similar reaction results have also been observed for the DMM carbonylation over acidic zeolite catalysts.12
The influence of CO partial pressures on the performance of DMM carbonylation over Nafion-H catalysts is studied from 9.8 to 29.6 atm. As displayed in Fig. 2, the increased pressure shows a beneficial impact on the conversion of DMM and the selectivity for MMAc. It should be noted that the actual pressures of CO in this vapour phase reaction system are considerably lower than that in the liquid phase carbonylation of formaldehyde,9 but a higher selectivity for the aimed product (MMAc) is obtained. Comparing with reactants concentration reported by Bell's group, the pressure of CO (29.6 atm) is higher. Nevertheless, the concentration of DMM is also considerably higher. The mole ratio (about 70) of CO to DMM in this reaction system is still lower than former reports.12,13 Higher reactants concentrations are beneficial for improving the manufacturing productivity. Additionally, the catalytic activity is found to be rather stable, higher than 50%, without obvious deactivation for 12 h on stream when the partial pressure of CO is 29.6 atm. However, the conversion of DMM decreases significantly from 35 to 25% at 9.8 atm. At lower CO pressure, the conversion of DMM is low. Thus, more unconverted DMM is favorable for the formation of the carbonaceous deposit, which covers the active site of the catalyst, leading to the gradual decrease in the reaction stability.
For the acid-catalyzed carbonylation of DMM, a Koch-type mechanism had been proposed and confirmed by in situ IR spectroscopy on acidic zeolite catalysts.13–15 From the DFT calculations, Bell and co-workers suggested that CO insertion between the methoxymethyl carbon and the framework oxygen atom to form the methoxyacetyl species was the rate-determining step in the carbonylation mechanism over acidic zeolites.14
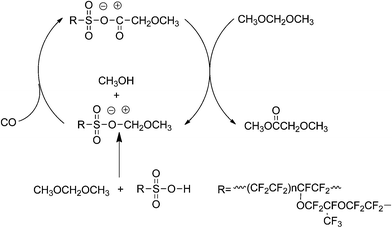
Based on the former work and experimental results, we deduce that the Koch-type mechanism also acts on the DMM carbonylation over the Nafion-H catalyst. Herein, a possible pathway for DMM carbonylation over Nafion-H has been proposed and illustrated in Scheme 1. First, the DMM carbonylation is triggered by reacting DMM with the sulfonic acid groups to form methanol and methoxymethyl species, which are attacked by CO to give the methoxyacetyl species. These intermediates further react with DMM molecules to produce MMAc and regenerate the methoxymethyl species. As known, higher partial pressure of CO can be considered equivalent to a higher concentration of CO. According to the proposed mechanism, a higher concentration of CO provides more opportunities to react with the methoxymethyl species, thereby resulting in better catalytic performance.
In order to demonstrate the outstanding catalytic performance, polystyrene sulfonic acid resins (PS–SO3–H), which have a similar –SO3H group, are chosen as references to compare with the Nafion-H catalyst. As shown in Fig. 3, it is notable that the Nafion-H resins exhibit considerably higher DMM conversion and selectivity for the desired product (MMAc) than the PS–SO3–H catalyst, even though the acid density of the PS–SO3–H catalyst is considerably higher than that of the Nafion-H catalyst (2.28 mmol g−1 vs. 0.504 mmol g−1). The space-time yield of MMAc over the Nafion-H resins catalyst at steady state (TOS ≈ 2 h) is more than three times (1.6 gMMAc gcata−1 h−1) higher than that over the PS–SO3–H catalyst (0.48 gMMAc gcata−1 h−1). Furthermore, Nafion-H resins show a higher conversion and selectivity for MMAc compared to H-FAU catalysts (Fig. SI1, see ESI†).
The excellent catalytic performance is assumably attributed to the structure and unique chemical properties of Nafion-H resins. It has been reported that the small pores of zeolites help promote the hydrogen-transfer step of the disproportionation of DMM, resulting in a decrease in the selectivity for MMAc.13 Compared to zeolite catalysts, Nafion-H resins have no small channels or cavities in which the disproportionation reactions mainly occur. Therefore, the disproportionation reaction of DMM is suppressed in the absence of steric constraint of the pore walls of zeolites. Moreover, the acid strength of solid acids plays an important role in the acid-catalyzed carbonylation reaction. Typically, the high acid strength of solid acids could promote carbonylation reactions.16,17 As references, various sulfonic acids with different acid strength have been loaded on porous silica supports to investigate the impact of the strength of acids on the reactivity of the DMM carbonylation. The reaction results are given in Table SI1 (see ESI†). Among all the sulfonic acids used in the present study, triflic acid, for which the Hammett value is −14.3, has the highest acid strength, and presents the highest rate of MMAc synthesis, up to 12.6 mol (mol H+)−1 h−1, while the rate of MMAc formation over the p-toluenesulfonic acid (Hammett value, 0.55) catalyst is less than 2 mol (mol H+)−1 h−1. These results clearly show that the high acid strength of catalysts is able to promote the carbonylation of DMM. Based on the above results, 1H MAS NMR of deuterated pyridine adsorbed on the catalysts is used to characterize the acid strength of solid acids and to elucidate the relation between the catalytic activity and the acid strength of catalysts. As proved by Zheng et al.,18 the stronger acid strength of solid acids leads to a smaller 1H chemical shift of pyridine ions in the range of 12–20 ppm. As displayed in Fig. 4, the 1H chemical shifts of adsorbed pyridine ions on Nafion-H and PS–SO3–H resin are 14.3 and 15.3 ppm, respectively. According to the report of Zheng et al., it can be concluded that the acid strength of the Nafion-H catalyst is considerably higher than that of PS–SO3–H resins. The high acid strength of Nafion-H resins is because of the powerfully electron-withdrawing –CF2 moieties directly attached to the sulfonic groups.19 The effect of the acid strength of solid acids on the activity of DMM carbonylation can be interpreted in light of the Koch reaction mechanism. As mentioned before, CO insertion reaction is the chemical rate-limiting step in the DMM carbonylation. During the CO insertion process, the methoxymethyl species partially dissociate from the adsorption sites to produce the carbenium ions and their conjugate anions at the transition states which are prevalent in the classic Koch mechanism.14,20–25 For the anions, the strongly inductive effect of –CF2 moieties and the mesomeric effect of sulfonate groups cause the delocalization of negative charges on the anions, hence stabilizing the anions, which reduces the activation energy for the carbonylation step. Consequently, a greater activity of the DMM carbonylation over the Nafion-H catalyst is observed. Additionally, Nafion-H catalysts show considerably better catalytic stability than PS–SO3–H resins.
 | ||
| Fig. 4 1H MAS NMR spectra of solid acid catalysts after adsorption of deuterated pyridine (1) Nafion-H, (2) polystyrene sulfonic acid resin. | ||
Conclusions
In summary, this study demonstrates that Nafion-H resins exhibit an excellent catalytic activity and selectivity in the vapour phase carbonylation of DMM. The excellent catalytic performance of the Nafion-H catalyst is attributed to the high acid strength which promotes the carbonylation reaction, and to its unique structure which helps suppress side reactions. Compared to other solid acid systems previously reported, this catalytic system, which is more efficient and selective, exhibits great potential for the industrial manufacture of EG.Notes and references
- H. Yue, Y. Zhao, X. Ma and J. Gong, Chem. Soc. Rev., 2012, 41, 4218 RSC.
- http://www.ptq.pemex.com/productosyservicios/eventosdescargas/Documents/Foro%20PEMEX%20Petroqu%C3%ADmica/2012/pci%20oxido%20de%20etileno.pdf.
- B. Li, S. Bai, X. Wang, M. Zhong, Q. Yang and C. Li, Angew. Chem., Int. Ed., 2012, 51, 11517 CrossRef CAS PubMed.
- Y. Sun, H. Wang, J. H. Shen, H. C. Liu and Z. M. Liu, Catal. Commun., 2009, 10, 678 CrossRef CAS PubMed.
- F. E. Celik, H. Lawrence and A. T. Bell, J. Mol. Catal. A: Chem., 2008, 288, 87 CrossRef CAS PubMed.
- Y. G. Kim, J. S. Lee and K. H. Lee, Res. Chem. Intermed., 1998, 24, 197 CrossRef CAS PubMed.
- D. J. Loader, U.S. Pat., 215285, 1939.
- A. T. Larson, U.S. Pat., 2153064, 1939.
- D. E. Hendriksen, Abstr. Pap. Am. Chem. Soc., 1983, 185, 176 Search PubMed.
- S. A. I. Barri and D. Chadwick, Catal. Lett., 2011, 141, 749 CrossRef CAS.
- J. F. Warker, U.S. Pat., 2529269, 1950.
- F. E. Celik, T.-J. Kim and A. T. Bell, Angew. Chem., Int. Ed., 2009, 48, 4813 CrossRef CAS PubMed.
- F. E. Celik, T.-J. Kim and A. T. Bell, J. Catal., 2010, 270, 185 CrossRef CAS PubMed.
- V. Shapovalov and A. T. Bell, J. Phys. Chem. C, 2010, 114, 17753 CAS.
- F. E. Celik, T. Kim, A. N. Mlinar and A. T. Bell, J. Catal., 2010, 274, 150 CrossRef CAS PubMed.
- S. D. Pirozhkov, A. S. Stepanyan, T. N. Myshenkova, M. B. Ordyan and A. L. Lapidus, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1982, 31, 1852 CrossRef.
- S. Y. Lee, J. C. Kim, J. S. Lee and Y. G. Kim, Ind. Eng. Chem. Res., 1993, 32, 253 CrossRef CAS.
- A. M. Zheng, H. L. Zhang, L. Chen, Y. Yue, C. H. Ye and F. Deng, J. Phys. Chem. B, 2007, 111, 3085 CrossRef CAS PubMed.
- M. A. Harmer and Q. Sun, Appl. Catal., A, 2001, 221, 45 CrossRef CAS.
- M. V. Luzgin, M. S. Kazantsev, W. Wang and A. G. Stepanov, J. Phys. Chem. C, 2009, 113, 19639 CAS.
- R. Gounder and E. Iglesia, J. Am. Chem. Soc., 2009, 131, 1958 CrossRef CAS PubMed.
- R. Gounder and E. Iglesia, Angew. Chem., Int. Ed., 2010, 49, 808 CrossRef CAS PubMed.
- A. Bhan and E. Iglesia, Acc. Chem. Res., 2008, 41, 559 CrossRef CAS PubMed.
- W. Wang and M. Hunger, Acc. Chem. Res., 2008, 41, 895 CrossRef CAS PubMed.
- R. Gounder and E. Iglesia, Acc. Chem. Res., 2012, 45, 229 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials preparation, characterization methods, additional figures. See DOI: 10.1039/c4ra06150k |
| This journal is © The Royal Society of Chemistry 2014 |