Effects of HRT and nitrite/ammonia ratio on anammox discovered in a sequencing batch biofilm reactor
Abstract
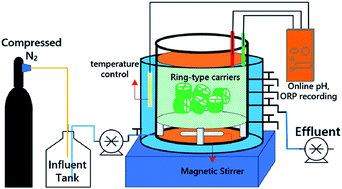
There are three key aspects of substrate effect on anaerobic ammonia oxidizing (anammox) bacteria: (1) substrate concentration-based nitrogen loading rate (NLR), (2) hydraulic retention time (HRT)-based NLR and (3) nitrite/ammonia ratio. The first part has been fully investigated in the past, while the latter two are still not properly understood. In this study, two types of substrate effects (HRT-based NLR and nitrite/ammonia ratio) were experimentally proved based on a 226 day operation of a sequencing batch biofilm reactor (SBBR) that was dominated by anammox bacteria. A modified first-order substrate removal kinetic model was developed, which efficiently fits to the experimental results. Decreasing HRTs from 72 h to 6 h were applied to the SBBR, and the HRT = 6 h was proven to be optimal for the highest nitrogen removal rate (NRR) (1.62 kg N m−3 d−1 and the total nitrogen removal efficiency >90%). In addition, the influent nitrite/ammonia ratio of 1.2 resulted in a stable and effective operation of anammox SBBR with an improved ammonia removal efficiency (by 17%) and an enhanced NRR (from 0.93 kg N m−3 d−1 to 1.14 kg N m−3 d−1).

 Please wait while we load your content...
Please wait while we load your content...