Electroless nanowelding of silver nanowires at room temperature
Abstract
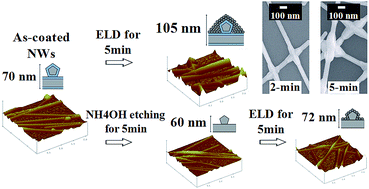
Silver nanowires (Ag NWs) using polyvinylpyrrolidone (PVP) surfactants were coated on either polyethylene terephthalate (PET) or glass substrates. The resistance of the Ag NW film was very high due to the PVP molecules wrapping around the Ag NWs and preventing good electrical contacts between them. We performed an Ag electroless deposition (ELD) on the Ag NW film at room temperature and achieved the welding of Ag NWs by enhancing the deposition of Ag right at the junctions of the two crossing wires. The welding of Ag NWs drastically reduced the sheet resistance (Rsh) of the film from 17 000 to 20 ohm sq−1 for only a 2 min treatment. By conducting the Ag ELD on the PVP-removed Ag NW film annealed at 200 °C, we showed that the PVP molecules concentrated at the junctions were responsible for the nanowelding behavior. In addition, both ethylenediamine (EDA) and ammonium hydroxide (NH4OH) could mildly etch the Ag NWs to remove PVP from the wires at room temperature, improving the contacts between Ag NWs and thus reducing the Rsh of the Ag NW film. An organic light emitting diode with ELD-treated Ag NW film was prepared to demonstrate the applicability of the nanowelding technique for conductivity enhancement. The better performance of the device based on ELD-treated Ag NW film compared with that on Ag thin film was due to the higher degree of light transmittance and work function of the Ag NWs.

 Please wait while we load your content...
Please wait while we load your content...