C(sp2)–C(sp2) cross coupling reactions catalyzed by an active and highly stable magnetically separable Pd-nanocatalyst in aqueous media†
Mohammad Ali Zolfigol*a,
Tahereh Azadbakhtab,
Vahid Khakyzadehac,
Razie Nejatyamid and
David M. Perrinb
aFaculty of Chemistry, Bu-Ali Sina University, Hamedan 6517838683, Iran. E-mail: zolfi@basu.ac.ir; mzolfigol@yahoo.com
bChemistry Department, UBC, 2036 Main Mall, Vancouver, BC, Canada V6T-1Z1. E-mail: T_azadbakht@yahoo.com; Dperrin@chem.ubc.ca
cInstitut fürOrganischeChemie, Albert-Ludwigs-Universitat, 79104 Freiburg, Germany. E-mail: mr.khakyzadeh@yahoo.com; v.khakyzadeh@basu.ac.ir
dChemistry Department, Faculty of Basic Science, Tarbiat Modares University, Tehran 14115-111, Iran. E-mail: sahba_r_nejat@yahoo.com
First published on 21st August 2014
Abstract
A new magnetite Pd-nanoparticle supported (4,5-diazafluoren-9-one)-derived palladium chloride (7) was synthesized, characterized and introduced. The nanocatalyst exhibited an efficient activity in Suzuki cross-coupling reactions with an environmentally-friendly (H2O/DMF) solvent system for 1–3 h at 100 °C and Mizoroki–Heck cross-coupling reactions. The catalyst can easily be recovered from the reaction system by using an external magnet and reused several times with high yields.
Introduction
Activity and recovery in catalysis science are two important parameters that have to be considered for the design of new catalysts. Nowadays, due to global sustainability and ecological problems, scientists in this field are taking a “green chemistry” approach whereby recovery and recycling are more desirable.1–3 Some types of catalysts have high activity but cannot be easily separated and reused in subsequent reactions. Hence, they do not satisfy the sustainability demands. A homogenous catalyst belongs to this category of catalysts—where the underlying issue is its inability to be recycled and hence homogenous catalysts are not acceptable from a “green chemist's” point of view.4Heterogeneous catalysts are a different type of catalyst that can be recycled and recovered but have low activity. Their low activity is attributed to the low surface contact that makes them inappropriate to use when in need of a perfect catalyst.5
Nanochemistry, as a novel field of research, enables us to scale down the size of particles and make nanoparticles with a variety of shapes and sizes with unique properties.6 Many types of nanoparticles have been synthesized for use as catalysts.7–13
One of the appealing advantages of nanoparticles is providing a high surface to volume ratio, which can increase the contact between catalysts and reactants. By increasing the contact between catalysts and reactants, one can improve the activity of the catalyst—a much desired parameter.14
The synthesis of magnetic nanoparticles that are small in size, highly active, and easy to separate with an external magnet are the ideal specifications of a catalyst. Such properties are achievable by employing nanochemistry technology.6,15
This type of catalysts that show high activity in addition to being easily recovered, separated, and reused in other reactions, will satisfy the sustainability and “green chemistry” demands. Especially Fe3O4 nanoparticles as a promising nanocatalyst have attracted many attentions because of their unique properties such as high surface to volume ratio, low toxicity and superparamagnetism.16–18
Palladium-based catalysts that are employed in Heck and Suzuki coupling reactions have shown high efficiency in the formation of C–C bonds.19–22 The resulting products play a significant role in the synthesis of bioactive compounds and organic products and are exploited in pharmaceutical industry applications.23,24
In this study, we demonstrated the production of Pd-catalyst based on magnetic Fe3O4 nanoparticles. We chose the Suzuki coupling reaction as a model reaction to test of our designed catalyst. The reaction was performed in environmentally-friendly solvent with high efficiency and excellent yields. We could easily separate the catalyst from the reaction medium with the external magnet and reuse it in other reactions. Described Pd-nanoparticles catalysts were also used as well as in Mizoroki–Heck coupling reaction as well.
Result and discussion
Fe3O4 particles (1) were synthesized according to the method reported previously by Qu et al.25 The particles were coated with a layer of silica with tetraethyl orthosilicate (TEOS). Silanation of the silica-coated magnetite nanoparticles (SMNPs) with 3-aminopropyltriethoxysilane gave compound (4). 4,5-diazafluoren-9-one (5) was synthesized26 and then were added to the solution. Reduction of imine groups by NaBH4 increases nanoparticles' stability in aqueous solution. Treatment of imine with water leads to hydrolysis back to the ketones and amine. Compound (6) was reacted with PdCl2 to achieve Palladium nanoparticles catalyst (7). These steps are illustrated in Fig. 1.Transmission electron microcopy (TEM) images of nanocatalyst are shown in Fig. 2. The size of the catalyst was approximately 35 nm. TEM images for the catalyst after five times recycling, are illustrated in Fig. 3.
Energy dispersive X-ray (EDX) analysis of the catalyst showed expected elements such as; iron, oxygen, silicon, carbon, nitrogen and palladium and spectrum indicated in Fig. 4 (EDX analysis of other layers inserted in ESI†). The percentage of the Pd was around 3.5 wt%.
Ultraviolet-visible spectroscopy (UV) at a range of 200–800 nm, was used to monitor changes in the reaction of 4,5-diazafluoren-9-one to amino-containing nanoparticles. We were able to observe the presence of an additional moiety onto the original structure of nanoparticles, specifically at λ = 251 nm. Fig. 5 illustrates the UV absorption curves of these compounds.27
First, the reaction between particles containing amino groups, such as compound (4) and 4,5-diazafluoren-9-one formed a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. Formation of this bond was identified with FT-IR spectra with an absorption band at 1634 cm−1 and the silica coated magnetite nanoparticles were confirmed by observation of abroad band at about 1083 cm−1 (Fig. 6).
N bond. Formation of this bond was identified with FT-IR spectra with an absorption band at 1634 cm−1 and the silica coated magnetite nanoparticles were confirmed by observation of abroad band at about 1083 cm−1 (Fig. 6).
Magnetic measurements of the samples were investigated by a vibrating sample magnetometer (VSM) at room temperature. Based on magnetization curves, the saturation of the obtained catalyst decreased from 60 emu g−1 (Fe3O4) to 7 emu g−1 for the catalyst. This drop in saturation accounts for the surface coating on Fe3O4 nanoparticles that interact with Pd-nanoparticles catalyst28 (Fig. 7).
Inductively coupled plasma optical emission spectroscopy (ICP/OES) analysis of the catalyst showed that the Pd content was about 4.3 wt% Pd in the systems.
Catalytic Suzuki reaction
To prove the activity of the new synthesized catalyst (7), the Pd nanoparticles catalyst was subjected to series of Suzuki reactions where the reaction of bromobenzene with phenylboronic acid was used as the model reaction. Different solvent, base and catalyst loading were investigated in these reactions.In the first trial, the coupling reaction was set up in the presence of DMF solvent, K2CO3 base and 0.2% catalyst loading (0.01 g) (at 100 °C for 3 hours (obtained yield was 96%). Water, H2O/DMF and toluene were investigated, and the best yields were observed with water/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as shown in Table 1. By testing different bases such as NaOAc, NaOH, Na2CO3 and CS2CO3, a decreasing in yield was observed (Table 1). The highest yield was obtained in the presence of 2% catalyst (0.01 g) (Table 1).
1) as shown in Table 1. By testing different bases such as NaOAc, NaOH, Na2CO3 and CS2CO3, a decreasing in yield was observed (Table 1). The highest yield was obtained in the presence of 2% catalyst (0.01 g) (Table 1).
| Entry | Solvent | Base | Pd (%) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition Bromobenzene (1 mmol), Phenylboronic acid (1.5 mmol), K2CO3 (2 mmol), solvent (4 mL).b Isolated yield. | |||||
| 1 | DMF | K2CO3 | 0.2 | 3 | 96 |
| 2 | DMF/H2O | K2CO3 | 0.2 | 3 | 96 |
| 3 | DMF/H2O | NaOH | 0.2 | 3 | 86 |
| 4 | DMF/H2O | NaOAc | 0.2 | 3 | 73 |
| 5 | DMF/H2O | Na2CO3 | 0.2 | 3 | 75 |
| 6 | DMF/H2O | CS2CO3 | 0.2 | 3 | 93 |
| 7 | H2O | K2CO3 | 0.2 | 3 | 56 |
| 8 | Toluene | K2CO3 | 0.2 | 3 | 60 |
| 9 | DMF/H2O | K2CO3 | 0.1 | 3 | 84 |
| 10 | DMF/H2O | K2CO3 | 0.3 | 3 | 96 |
| 11 | DMF/H2O | K2CO3 | 0.2 | 1.5 | 80 |
The obtained optimized reaction conditions are presented in Table 1. The reaction was then investigated with different aryl halides and arylboronic acids at the determined optimized conditions and the results are summarized in Table 2. We used aryl iodide to test the catalytic activity of the Pd nanoparticles catalyst.
| Entry | Aryl halides | Aryl boronic acids | Time (h) | Yieldb (%) |
|---|---|---|---|---|
a Reaction condition Bromobenzene (1 mmol), Phenylboronic acid (1.5 mmol), K2CO3 (2 mmol), DMF/H2O (4 mL, v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Pd catalyst (0.2 mol%), 100 °C.b Isolated yield. 1), Pd catalyst (0.2 mol%), 100 °C.b Isolated yield. |
||||
| 1 |  |
 |
1 | 96 |
| 2 |  |
 |
1 | 96 |
| 3 |  |
 |
1 | 93 |
| 4 | 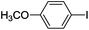 |
 |
1 | 96 |
| 5 |  |
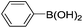 |
3 | 96 |
| 6 |  |
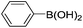 |
3 | 95 |
| 7 |  |
 |
3 | 95 |
| 8 |  |
 |
3 | 98 |
| 9 |  |
 |
3 | 98 |
| 10 |  |
 |
3 | 89 |
| 11 |  |
 |
1 | 98 |
| 12 |  |
 |
3 | 97 |
| 13 |  |
 |
6 | 12 |
| 14 |  |
 |
3 | 92 |
| 15 |  |
 |
3 | 84 |
The time of reaction for aryl iodide was shorter than aryl bromides (1 h) and very good to excellent yields were obtained. It should be noted that the yield of the reaction for aryl bromides bearing an electron-withdrawing group was a little more than electron-releasing group (Table 2, entries 8–9). We investigated aryl chloride and unfortunately a low yield was observed. We utilized this catalyst in coupling 2-naphthylboronicacid as a larger ring and it was successful (entries 14–15).
The ability to recover and reuse is another aspect that we investigated for this new catalyst. The reaction of phenyl boronic acid and bromobenzeneas the model was chosen. After separating the catalyst from the reaction by an external magnet, it was washed, dried and then directly carried forward to the next reaction. We repeated this recycling and reusing of the catalyst twenty two times and the yields of reactions are shown in Table 3. Reusability of the Pd magnetically separable nanocatalyst is shown in Fig. 8. A slight decrease in the catalytic activity was observed after these reactions. Metal leaching of the catalyst was studied before and after the reaction by ICP-OES analysis. The Pd content was found to be 4.3 wt% and 4.01 wt% before and after five times reactions series respectively, which was verified to imply insignificant Pd leaching.
So that know whether the reaction takes place at a heterogeneous system or Pd-leached species acts as a homogeneous catalyst, the hot filtration test was carried out at 100 °C in a similar way to that previously reported.14
The Suzuki cross-coupling reaction for bromobenzene and phenyl boronic acid in the presence of the catalyst in optimized condition was cooled down to room temperature after 20 min. Isolated yield of reaction product showed that 10% of bromobenzene has been converted to its corresponding coupled product. The catalyst was filtered by a magnet and the obtained filtrate was left to react more at mentioned temperature. The result of reaction after 12 h showed no progressive. Comparison of this result with the data of Table 2, entry 5 (96% isolated yield after 3 h), indicates that the reaction has been catalyzed mainly by the heterogeneous system.
A schematic representation of the proposed catalytic route for Suzuki reaction has been presented in Scheme 1.
In a first step, the active palladium catalyst reacts with the arylhalide (oxidative addition) to produce Pd(II) intermediate. Then, aryl moiety of phenyl boronic acid exchange with halide and finally, these complexes undergo reductive elimination to afford the expected product.29–31
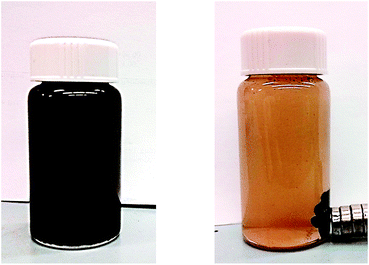
Since the color of the Pd-nanoparticles catalyst changed from brown to black upon usage in the coupling reaction. X-ray photoelectron spectroscopy (XPS) helped determine the oxidation state of the Pd surface in the catalyst. According to this variation in color, we performed XPS on both samples. The binding energy curve showed a double peak at 337.5 eV (3d5/2) and 342 eV (3d3/2) that could be attributed to Pd(II) for the brown catalyst. The same measurement for the black sample showed different values for double peaks, 335.2 (3d5/2) and 340.2 eV (3d3/2) that related to Pd (0). These results are consistent with previously reported values in literature32,33 (Fig. 9).
Catalytic Heck reaction
The Pd-nanoparticles catalyst were also used for a good rang of Heck reactions where the reaction of iodobenzene with vinylbenzene (phenylethene) was used as the model reaction. Various solvent, base, temperature and catalyst loading were investigated in mentioned reactions. The obtained optimized reaction conditions are presented in Table 4.| Entry | Base | Catalyst (g) | Solvent | T (°C) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|---|
| a Isolated yield. | ||||||
| 1 | DABCO | 0.05 | DMSO | 140 °C | 3 | 86 |
| 2 | DABCO | 0.05 | DMF | 140 °C | 3 | 81 |
| 3 | DABCO | 0.05 | Toluene | Reflux | 24 | Trace |
| 4 | DABCO | 0.05 | H2O | Reflux | 24 | 32 |
| 5 | DABCO | 0.05 | EtOH | Reflux | 24 | 25 |
| 6 | DABCO | 0.05 | Solvent free | 130 °C | 1 | 84 |
| 7 | DABCO | 0.03 | Solvent free | 140 °C | 60 min | 80 |
| 8 | DABCO | 0.05 | Solvent free | 140 °C | 45 min | 91 |
| 9 | DABCO | 0.07 | Solvent free | 140 °C | 45 min | 91 |
| 10 | — | 0.05 | Solvent free | 140 °C | 24 | — |
| 11 | Et3N | 0.05 | Solvent free | 140 °C | 1 | 40 |
| 12 | NaOH | 0.05 | Solvent free | 140 °C | 3 | 58 |
| 13 | K2CO3 | 0.05 | Solvent free | 140 °C | 3 | 64 |
| 14 | KOAc | 0.05 | Solvent free | 140 °C | 3 | 71 |
The reaction was then investigated with different aryl halides with styrene and n-butyl acrylate esters at the determined optimized conditions and the results are summarized in Table 5.
| Entry | ArX | Y | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction condition Pd-nanocatalysts (0.05 g), aryl halide (1 mmol), n-butyl acrylate (1.5 mmol) or styrene (1.5 mmol) and DABCO (1.5 mmol).b Isolated yield. | ||||
| 1 | 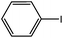 |
Ph | 45 min | 91 |
| 2 |  |
Ph | 12 | 78 |
| 3 |  |
Ph | 1.5 | 84 |
| 4 | 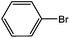 |
Ph | 55 min | 93 |
| 5 | 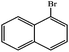 |
Ph | 24 | 82 |
| 6 | 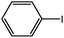 |
-CO2Bu-n | 2 | 92 |
| 7 |  |
-CO2Bu-n | 6 | 90 |
| 8 | 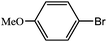 |
-CO2Bu-n | 24 | 68 |
| 9 |  |
-CO2Bu-n | 2 | 85 |
| 10 |  |
-CO2Bu-n | 24 | 88 |
Conclusion
In this research, new magnetite nanoparticles supported (4,5-diazafluoren-9-one)-derived palladium chloride (7) was synthesized, characterized and introduced. The described pd-nanocatalysts were observed to be stable and applied in high catalysts activity in Suzuki cross-coupling reactions with a H2O/DMF solvent system and Mizoroki–Heck cross-coupling reactions. The catalyst can easily be recovered from the reaction mixture by using an external magnet and reusing it several times with high yields. Catalyst was recycled and reused for several times with any loosing of catalytic activity.Experimental section
Materials
FeCl3 (anhydrous), Na2SO3, (3-amino propyl) triethoxysilane, phenatroline PdCl2 were purchased from Alfa Aesar and used without any purification.Characterization methods
The structure of the new magnetite nanocatalyst was characterized by FT-IR, UV-VIS, EDX, TEM, XPS and VSM analysis. FTIR-spectra was recorded by Perkin Elmer PE-1600-FTIR. Pd content of the catalyst was determined by inductively coupled plasma (ICP) ICP-OES. UV study was performed by UV photodiode array. X-ray photoelectron spectroscopy (XPS) was carried out by Dual anode (Mg and Al K alpha) achromatic X-ray source.The size of the magnetite nanoparticles was measured by using transmission electron microscope (Hitachi H 7600 TEM). Magnetic measurement of materials was investigated with a vibrating sample magnetometer (VSM - 4 inch, Daghigh Meghnatis Kashan Co., Kashan, Iran) at room temperature.
Catalyst preparation
At first magnetic nanoparticles Fe3O4 and Fe3O4@SiO2 were synthesized according to the procedure previously reported in literature.22,28,34Silanation of the Fe3O4@SiO2 magnetite nanoparticles
Initially silica coated nanoparticles put under vacuum to dry. 2 g of dry particles was dispersed in 100 mL dry toluene and 30 min was sonicated. 7.5 mL (32.04 mmol) of (3-aminopropyl) triethoxysilane was added dropwise to the suspended solid nanoparticles under mechanical stirring. The reaction was refluxed at 115 °C for 20 h. After refluxing, the nanoparticles were cooled down and then separated by a magnet. The nanoparticles were then washed three times with anhydrous methanol and then dried under vacuum. Next, a back titration was performed to determine the concentration of amines, which was measured to be 0.048–0.502 mmol g−1.General procedure for the preparation of 4,5-diazafluoren-9-one
According to the method reported by Mazaleyrat et al.,26 a boiling solution of phenanthroline (2.5 g) and KOH (1.3 g) in water (130 mL) was prepared. A hot solution of KMnO4 (6.4 g in 100 mL water) was added to the solution dropwise over 2 h and then refluxed for 1 h. The hot solution was filtered and left overnight at room temperature. The primary yellow crystal was obtained and recrystallized in water.General procedure for the preparation of SMNPs-supported 4,5-diazafluoren-9-one (SMNPs-DF)
1 g of silanated nanoparticles was suspended in 10 mL of dry methanol and sonicated for 30 min. Then, a solution of 0.1 g (0.52 mmol) 4,5-diazafluoren-9-one in 5 mL dry methanol was added in dropwise motion to the nanoparticles and then refluxed for 48 h under argon. After 48 h, the mixture was cooled to room temperature, and then 0.0197 g (0.52 mmol) of NaBH4 was added. The mixture was then heated to 40 °C for 24 h. The nanoparticles were washed (3 × 10 mL) with methanol and then dried under vacuum.General procedure to preparation of palladium catalyst
1 g of dry nanoparticles from the final step and 15 mL dry toluene was sonicated. Then, PdCl2 (0.6 mmol, 0.1 g) in 15 mL toluene was added and the mixture was refluxed for 12 h under argon. The resulting product containing an external magnet was separated, subjected to a water wash (1 × 10 mL), methanol wash (3 × 10 mL) and then dried under vacuum.General procedure for Suzuki reaction
A mixture of aryl halide (1 mmol), arylboronic acid (1.5 mmol), K2CO3 (2.0 mmol), DMF/H2O as solvent (4.0 mL, v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the catalyst (0.01 g) was added to a round bottom flask. The mixture of reaction was stirred at 100 °C, in an air atmosphere for the required time to complete the reaction. When the reaction was completed, the reaction was cooled and added 25 mL Et2O to the solution, the organic phase was washed with water (2 × 10 mL) and evaporated the solvent. The residual of reaction was purified by flash chromatography to provide the pure coupling products. The catalyst separated magnetically by using external magnet. The catalyst was washed with H2O, Et2O and recovered.
1) and the catalyst (0.01 g) was added to a round bottom flask. The mixture of reaction was stirred at 100 °C, in an air atmosphere for the required time to complete the reaction. When the reaction was completed, the reaction was cooled and added 25 mL Et2O to the solution, the organic phase was washed with water (2 × 10 mL) and evaporated the solvent. The residual of reaction was purified by flash chromatography to provide the pure coupling products. The catalyst separated magnetically by using external magnet. The catalyst was washed with H2O, Et2O and recovered.
General procedure for Mizoroki–Heck reaction
To a flask, a mixture of Pd-nanocatalysts (0.05 g), aryl halide (1 mmol), n-butyl acrylate (1.5 mmol) or styrene (1.5 mmol) and DABCO (1.5 mmol) were added and heated at 140 °C under solvent-free conditions. After the completion of the reaction as monitored by TLC analysis, ethylacetate (10 mL) was added to the flask. The magnetic nanoparticles were separated by absorbing on the magnetic stirring bar. Water (3 × 15 mL) was added to the ethylacetate phase and decanted. The organic layer was dried over anhydrous Na2SO4. After evaporation of the solvent, the resulted crude products were purified by column chromatography (hexane/ethylacetate) giving the pure products in excellent yields.Acknowledgements
The authors gratefully acknowledge the Bu-Ali Sina and Research Council, University of British Columbia and Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS), for providing support to this work.References
- J. a. Gladysz, Pure Appl. Chem., 2001, 73, 1319–1324 CrossRef.
- J. Grunes, J. Zhu and G. a. Somorjai, Chem. Commun., 2003, 2257–2260 RSC.
- G. Lv, W. Mai, R. Jin and L. Gao, Synlett, 2008, 2008, 1418–1422 CrossRef.
- F. Cozzi, Adv. Synth. Catal., 2006, 348, 1367–1390 CrossRef CAS.
- W. Teunissen, A. A. Bol and J. W. Geus, Catal. Today, 1999, 48, 329–336 CrossRef CAS.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed. Engl., 2010, 49, 3428–3459 CrossRef CAS.
- Z. Wang, P. Xiao, B. Shen and N. He, Colloids Surf., A, 2006, 276, 116–121 CrossRef CAS PubMed.
- A. Schätz, M. Hager and O. Reiser, Adv. Funct. Mater., 2009, 19, 2109–2115 CrossRef.
- S. Wittmann, A. Schätz, R. N. Grass, W. J. Stark and O. Reiser, Angew. Chem., Int. Ed. Engl., 2010, 49, 1867–1870 CrossRef CAS PubMed.
- J. Mondal, T. Sen and A. Bhaumik, Dalton Trans., 2012, 41, 6173–6181 RSC.
- X. Jin, K. Zhang, J. Sun, J. Wang, Z. Dong and R. Li, Catal. Commun., 2012, 26, 199–203 CrossRef CAS.
- A. R. Moosavi-Zare, M. A. Zolfigol, V. Khakyzadeh, C. Böttcher, M. H. Beyzavi, A. Zare, A. Hasaninejad and R. Luque, J. Mater. Chem. A, 2014, 2, 770 CAS.
- M. A. Zolfigol, A. R. Moosavi-Zare, P. Moosavi, V. Khakyzadeh and A. Zare, C. R. Chim., 2013, 16, 962–966 CrossRef CAS PubMed.
- M. A. Zolfigol, V. Khakyzadeh, A. R. Moosavi-Zare, A. Rostami, A. Zare, N. Iranpoor, M. H. Beyzavi and R. Luque, Green Chem., 2013, 15, 2132 RSC.
- A. Alizadeh, M. M. Khodaei, M. Beygzadeh, D. Kordestani and M. Feyzi, Bull. Korean Chem. Soc., 2012, 33, 2546–2552 CrossRef CAS.
- Y.-S. Lin and C. L. Haynes, Chem. Mater., 2009, 21, 3979–3986 CrossRef CAS.
- Y. Deng, Y. Cai, Z. Sun, J. Liu, C. Liu, J. Wei, W. Li, C. Liu, Y. Wang and D. Zhao, J. Am. Chem. Soc., 2010, 132, 8466–8473 CrossRef CAS.
- L. M. Rossi, I. M. Nangoi and N. J. S. Costa, Inorg. Chem., 2009, 48, 4640–4642 CrossRef CAS.
- P. D. Stevens, J. Fan, H. M. R. Gardimalla, M. Yen and Y. Gao, Org. Lett., 2005, 7, 2085–2088 CrossRef CAS.
- M. Zhu and G. Diao, J. Phys. Chem., 2011, 24743–24749 CAS.
- B. Karimi and D. Enders, Org. Lett., 2006, 8, 1237–1240 CrossRef CAS.
- D. Elhamifar, B. Karimi, J. Rastegar and M. H. Banakar, ChemCatChem, 2013, 5, 2418–2424 CrossRef CAS.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 2457–2483 CrossRef CAS.
- A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed. Engl., 2002, 41, 4176–4211 CrossRef CAS.
- S. Qu, H. Yang, D. Ren, S. Kan, G. Zou, D. Li and M. Li, J. Colloid Interface Sci., 1999, 215, 190–192 CrossRef CAS.
- J. Mazaleyrat, K. Wright, M. Wakselman, F. Formaggio, M. Crisma and C. Toniolo, Eur. J. Org. Chem., 2001, 1821–1829 CrossRef CAS.
- G. a. Shabir, J. Chromatogr. Sci., 2008, 46, 643–648 CAS.
- H. Zhang and G. Zhu, Appl. Surf. Sci., 2012, 258, 4952–4959 CrossRef CAS.
- H. Firouzabadi, N. Iranpoor and F. Kazemi, J. Mol. Catal. A: Chem., 2011, 348, 94–99 CrossRef CAS.
- H. Firouzabadi, N. Iranpoor, F. Kazemi and M. Gholinejad, J. Mol. Catal. A: Chem., 2012, 357, 154–161 CrossRef CAS.
- H. Firouzabadi, N. Iranpoor and A. Ghaderi, J. Mol. Catal. A: Chem., 2011, 347, 38–45 CrossRef CAS.
- A. Khalafi-Nezhad and F. Panahi, Green Chem., 2011, 13, 2408 RSC.
- S. R. Sanjaykumar, B. D. Mukri, S. Patil, G. Madras and M. S. Hegde, j. chem. sci, 2011, 123, 47–54 CrossRef CAS.
- N. Koukabi, E. Kolvari, A. Khazaei, M. A. Zolfigol, B. Shirmardi-Shaghasemi and H. R. Khavasi, Chem. Commun., 2011, 47, 9230–9232 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06097k |
| This journal is © The Royal Society of Chemistry 2014 |