Binding interaction of a newly developed bisindole drug molecule with α-cyclodextrin: face to face shielding of indole hoops
Abstract
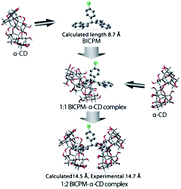
Binding interactions of a newly developed drug molecule namely 3,3′-bis(indolyl)-4-chlorophenylmethane (BICPM) with α-cyclodextrin have been studied using steady state and picosecond time resolved fluorometric techniques. A significant increase both in steady state anisotropy (r) and in the average rotational correlation time in the CD environments compared with that in a pure aqueous phase indicates that the rotational dynamics of BICPM are substantially slowed down upon binding with the α-cyclodextrin. Critical spectral analysis reveals the formation of two types of inclusion complex between the fluorophore and α-cyclodextrin (α-CD) depending on the relative population of the two. The stoichiometries and association constants of these complexes have been determined by monitoring the fluorescence data. Hydrodynamic radii of the formed 1 : 2 probe-α-cyclodextrin supramolecular complex have also been determined. From the determined hydrodynamic radii and from molecular docking analysis it is argued that probably two CD cavity shields the indole moiety in a face-to-face manner.

 Please wait while we load your content...
Please wait while we load your content...