Phosphorus-containing polymers from tetrakis-(hydroxymethyl)phosphonium sulfate iii. A new hydrolysis-resistant tris(allyloxymethyl)phosphine oxide and its thiol-ene reaction under ultraviolet irradiation†
Abstract
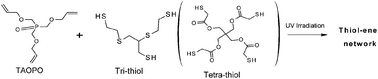
Two kinds of new phosphorus-containing crosslinked polymer materials were prepared via thiol-ene photopolymerization and their properties were studied. In order to prepare these crosslinked polymer materials, multifunctional monomer tris(allyloxymethyl)phosphine oxide (TAOPO) was synthesized from an eco-friendly raw material, tetrakis(hydroxymethyl) phosphonium sulfate (THPS). Crosslinked poly(phosphine oxide) networks were then produced by a thiol-ene reaction in which TAOPO reacts with the two kinds of polythiol under ultraviolet (UV) irradiation. The new crosslinked polymers possess long-term hydrolysis-resistance property because the monomer TAOPO contains a phosphorus–carbon bond. The crosslinked polymers have a high gel content, high dielectric constant, low dielectric loss, and excellent transparency with a high refractive index and high Abbe number. DMA and TGA results indicated that all cured poly(phosphine oxide) were uniform networks and exhibited a high thermal stability.

 Please wait while we load your content...
Please wait while we load your content...