Superior electrochemical performances of double-shelled CuO yolk–shell powders formed from spherical copper nitrate–polyvinylpyrrolidone composite powders†
Young Jun Hong and
Yun Chan Kang*
Department of Materials Science and Engineering, Korea University, Anam-dong, Seongbuk-gu, Seoul 136-713, Republic of Korea. E-mail: yckang@korea.ac.kr; Fax: +82-2-928-3584; Tel: +82-2-3290-3268
First published on 21st October 2014
Abstract
Spherical copper nitrate–polyvinylpyrrolidone (PVP) composite powders coated with a copper nitrate hydroxide [Cu2(OH)3NO3]–carbon composite are prepared by a one-pot spray pyrolysis process. In this, Cu2(OH)3NO3 and carbon are formed by dehydration of copper nitrate and carbonization of PVP, respectively. Thermal decomposition of the composite powders is then performed at 300 °C under an air atmosphere, producing the final yolk–shell-structured CuO powders. The electrochemical properties of these powders are then compared with those of commercial CuO nanopowders. The discharge capacities of the CuO yolk–shell powders and the commercial CuO nanopowders after 240 cycles at a current density of 500 mA g−1 are 590 and 302 mA h g−1, respectively. Furthermore, the discharge capacity of the CuO yolk–shell powders is as high as 615 mA h g−1, even after 1000 cycles at a current density of 1000 mA g−1. Electrochemical impedance spectroscopy reveals that the structural stability of the CuO yolk–shell powders during cycling lowers the charge transfer resistance, and thereby improves the lithium ion diffusion rate.
Introduction
Recently, yolk–shell-structured materials with a distinctive core@void@shell configuration have been actively studied for their potential use in lithium ion batteries (LIBs) as anode and cathode materials.1–15 Yolk–shell-structured metal oxides in particular have demonstrated great potential as anode materials, as the void space afforded between the yolk and shell creates a buffer for the large volume change that occurs in the structure of metal oxides during Li+ insertion and extraction, which would otherwise strongly affect its electrochemical properties.5–15 In addition, this yolk–shell structure shortens the diffusion length and increases the electrolyte–electrode contact area for Li+ insertion/extraction. As a result, electrodes produced using metal oxide yolk–shell powders typically exhibit good electrochemical properties at high current densities.5–15Copper oxide (CuO) has been considered a particularly promising candidate for use as a battery material because of its low cost, chemical stability, nontoxic nature, and high theoretical capacity (674 mA h g−1); however, it suffers from a poor cyclability and low capacity at high current densities, which is mainly caused by its inherently low conductivity and morphological collapse due to a large volume expansion during cycling.16–24 In an attempt to improve these electrochemical properties, many previous studies have focused on the preparation of nanostructured CuO materials, such as nanoparticle, nanocube, nanorod, hollow, mesoporous, and etc.16–31 However, despite this diversity in the structures that have been studied, the preparation of yolk–shell-structured CuO materials and their electrochemical properties have been scarcely studied.16
Spray pyrolysis, which represents one of the scalable gas-phase reaction methods, has recently been successfully applied to the preparation of yolk–shell-structured materials of various compositions.3,4,12–15 In these previous studies, the desired structure was achieved by the combustion of a metal oxide–carbon composite intermediate product; and as such, the sucrose used as a carbon source played a key role in its preparation. However, a sucrose additive dissolved in a spray solution is not appropriate for the preparation of CuO yolk–shell powders by spray pyrolysis. Heat evolution by composition of carbon formed by polymerization of sucrose precluded the formation of yolk–shell structure of CuO. Furthermore, the formation of a yolk–shell structure is impeded by Ostwald ripening and crystallization of CuO, even at low temperatures. This study therefore proposes a new means of producing yolk–shell powders via spray pyrolysis, in which spherical composite powders of a metal salt and polyvinylpyrrolidone (PVP) are first prepared by a low temperature spray pyrolysis process. This is then followed by decomposition in a box furnace under an air atmosphere, resulting in a metal oxide yolk–shell powder. In this way, yolk–shell-structured CuO powders were successfully prepared and their formation mechanism was investigated. In addition, the electrochemical properties of these CuO yolk–shell powders were evaluated with regards to their potential use as anode materials for LIBs and compared with those of commercial CuO nanopowders.
Experimental
Material fabrication
A spray-pyrolysis process was used to prepare the Cu nitrate–PVP composite coated with a copper nitrate hydroxide [Cu2(OH)3NO3]–carbon composite powders (precursor of yolk–shell-structured powders). The aqueous spray solution was prepared using copper nitrate trihydrate [Cu(NO3)2·3H2O] and PVP (Mw = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The total concentration of the Cu components dissolved in distilled water was 0.2 M. The 10 g of PVP were dissolved in 250 mL spray solution. The preparation temperature was 300 °C, and the carrier gas was air with 20 L min−1. The precursor powders obtained by spray pyrolysis were post-treated at 200 and 300 °C under air atmosphere.
000). The total concentration of the Cu components dissolved in distilled water was 0.2 M. The 10 g of PVP were dissolved in 250 mL spray solution. The preparation temperature was 300 °C, and the carrier gas was air with 20 L min−1. The precursor powders obtained by spray pyrolysis were post-treated at 200 and 300 °C under air atmosphere.
Characterization
The crystal structures of the powders were investigated using X-ray diffractometry (XRD, Rigaku DMAX-33) using Cu Kα radiation (λ = 1.5418 Å) at the Korea Basic Science Institute (Daegu). The morphologies of the obtained powders were characterized using scanning electron microscopy (SEM, JEOL JSM-6060) and high-resolution transmission electron microscopy (TEM, JEOL JEM-2010). The Brunauer–Emmett–Teller (BET) surface areas of the powders were measured using nitrogen gas as the adsorbate.Electrochemical measurements
The capacities and cycle properties of the CuO yolk–shell powders and the commercial CuO nanoparticles were measured using 2032-type coin cells. The electrodes were prepared using a slurry consisting of 70 wt% active anode material, 20 wt% carbon black (Super-P) as a conductive material, and 10 wt% binder composed of sodium carboxymethyl cellulose (CMC) on copper foil. Lithium metal and a microporous polypropylene film were used as the counter electrode and separator, respectively. Lithium hexafluorophosphate (1 M) in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio with 2 wt% vinylene carbonate (VC) was used as the electrolyte. The entire cell was assembled under an argon atmosphere in a glove box. The charge/discharge characteristics of the samples were measured at various current densities in the voltage range 0.01–3.0 V. Cyclic voltammetry measurements were carried out at a scan rate of 0.1 mV s−1 between 0.01 and 3 V. Electrochemical impedance spectra of the yolk–shell-structured CuO powders and the commercial CuO nanoparticles were analyzed in the frequency range between 100 kHz and 10 MHz at room temperature with a signal amplitude of 5 mV.
1 volume ratio with 2 wt% vinylene carbonate (VC) was used as the electrolyte. The entire cell was assembled under an argon atmosphere in a glove box. The charge/discharge characteristics of the samples were measured at various current densities in the voltage range 0.01–3.0 V. Cyclic voltammetry measurements were carried out at a scan rate of 0.1 mV s−1 between 0.01 and 3 V. Electrochemical impedance spectra of the yolk–shell-structured CuO powders and the commercial CuO nanoparticles were analyzed in the frequency range between 100 kHz and 10 MHz at room temperature with a signal amplitude of 5 mV.
Results and discussion
The schematic diagram depicting the formation mechanism of a CuO yolk–shell powder is shown in Scheme 1. In this, the Cu nitrate–PVP composite powder is prepared from a spray solution containing Cu nitrate and PVP by means of one-pot spray pyrolysis at 300 °C; the dehydration of the copper nitrate and carbonization of the PVP occurring near the surface of the composite powders. As a result, a Cu nitrate–PVP composite coated with a copper nitrate hydroxide [Cu2(OH)3NO3]–carbon composite was prepared directly by spray pyrolysis. Copper nitrate hydroxide is a basic Cu(II) salt with a layered structure. The prepared composite powder had a spherical shape and non-aggregation characteristics. Moreover, the ivory color of the prepared composite powders reveals the non-decomposition of copper nitrate and PVP in the spray pyrolysis process. The Cu2(OH)3NO3–carbon composite covering the Cu nitrate–PVP composite powder exhibited the nanostructure; the coating layer being an aggregate of Cu2(OH)3NO3 nanorods uniformly coated by carbon as shown in Scheme 1. The Cu nitrate–PVP composite powders were transformed into CuO yolk–shell powders by post-treatment inside a box furnace under an air atmosphere through several step changes, as described in Scheme 1. First, decomposition of the Cu2(OH)3NO3–carbon composite coating layer produces a core–shell-structured powder of Cu nitrate–PVP/CuO. Melting of the undecomposed inner part of the powder during this decomposition resulted in a yolk–shell powder with a configuration of Cu nitrate–PVP@void@CuO. Second, decomposition of the surface of the melted Cu nitrate–PVP core produced a powder with a configuration of Cu nitrate–PVP/CuO@void@CuO. Finally, the remelting and subsequent decomposition of the remaining Cu nitrate–PVP resulted in a double-shelled yolk–shell powder with the configuration of CuO@void@CuO@void@CuO.The formation mechanism of the CuO yolk–shell powder was investigated through the various morphology changes in the Cu nitrate–PVP composite powders according to the post-treatment temperatures. Fig. 1 shows the morphologies and dot-mapping images of the powders directly prepared by spray pyrolysis process, which reveals a monoclinic-phase crystal structure of copper nitrate hydroxide [Cu2(OH)3NO3, JCPDS file no. 74-1749], as shown in Fig. S1.† This Cu2(OH)3NO3 is formed by the dehydration of hydrated copper nitrate, which was incomplete at a preparation temperature of 300 °C because of the short residence time of the powders inside the reactor. Consequently, the resulting powders are a composite of copper nitrate hydroxide, hydrated copper nitrate, and PVP. The SEM images in Fig. 1a reveals the spherical, filled, and non-aggregated morphology of the as-prepared powders directly obtained by spray pyrolysis. However, the SEM and TEM images as shown in Fig. 1b and c obtained after dissolution in water reveal the hollow structure and low shell thickness of the composite powders. Dissolution of water-soluble hydrated copper nitrate and PVP occurred during the washing process; and as result, the Cu nitrate–PVP composite coated with copper nitrate hydroxide [Cu2(OH)3NO3]–carbon composite powders were transformed into hollow-structured Cu2(OH)3NO3–carbon composite powders after dissolution in distilled water. The TEM images in Fig. 1c and d show the detailed structure of these Cu2(OH)3NO3–carbon composite powders, comprising an aggregate of needle-like Cu2(OH)3NO3 nanocrystals covered with amorphous carbon, as shown by the arrows. The dot-mapping images of the composite powders, as shown in Fig. 1e, demonstrates uniform distributions of Cu, C, and N. Nitrogen and copper components were originated from the Cu2(OH)3NO3. Fig. S2† depicts the thermogravimetric (TG) curve of the copper nitrate–PVP composite powders coated with Cu2(OH)3NO3–carbon layer. This TG curve shows distinct two weight losses below 500 °C: the first weight loss near 120 °C occurring due to dehydration of hydrated copper nitrate to form Cu2(OH)3NO3; while the second weight loss observed at temperatures between 140 and 300 °C is due to the decomposition of Cu2(OH)3NO3, PVP, and carbon. The total weight loss of the composite powders below 500 °C was 75 wt%.
Fig. 2 shows the morphologies and dot-mapping images of the powders post-treated at 200 °C, with Fig. 2e revealing the core–shell structure of the powder and the presence of Cu, N, and C components in the inner regions. However, the presence of carbon was not observed in the outer regions of the powder. In the XRD pattern shown in Fig. S1,† the powders are revealed to have a mixed crystal structure of Cu2O and CuO. Complete decomposition of Cu2(OH)3NO3 into copper oxides occurred. The TEM images of the powders show a clear core–shell structure with a filled morphology, as shown by the dotted circles in Fig. 2b and c. The EDX spectrum of the powders post-treated at 200 °C, as shown in Fig. S3,† shows a nitrogen component that is attributable to the PVP. The inset images of Fig. 2d show the crystalline structure of the powders, in which can be seen clear lattice fringes separated by 0.23 nm that correspond to the (111) crystal plane of CuO. These results indicate that the complete decomposition of Cu2(OH)3NO3 into Cu2O and CuO occurs at a post-treatment temperature of 200 °C and that the decomposition of carbon near the powder surface resulted in core–shell-structured powders with a configuration of CuO–Cu2O–PVP/CuO.
 | ||
| Fig. 2 Morphologies and dot-mapping images of the powders post-treated at 200 °C: (a) SEM image, (b)–(d) TEM images, and (e) dot-mapping images. | ||
Fig. 3 shows the morphologies of the CuO powders post-treated at 300 °C. Both the SEM and TEM images show the corrugated structure of the CuO powders, whereas the TEM images in Fig. 4b and c also clearly show a yolk–shell structure. In the case of the powder shown in Fig. 3c, this structure consisted of three shells, as indicated by arrows. The SAED pattern with clear rings and the high resolution TEM image shown in Fig. 3d reveal the highly crystalline structure of the CuO powders post-treated at 300 °C. Furthermore, no carbon component could be observed in the dot-mapping images shown in Fig. 3e. It is therefore determined that these yolk–shell-structured CuO powders were formed by step-by-step decompositions of Cu nitrate–PVP composite powders coated with Cu2(OH)3NO3–carbon composite; the BET surface area of the CuO yolk–shell powders being 18 m2 g−1.
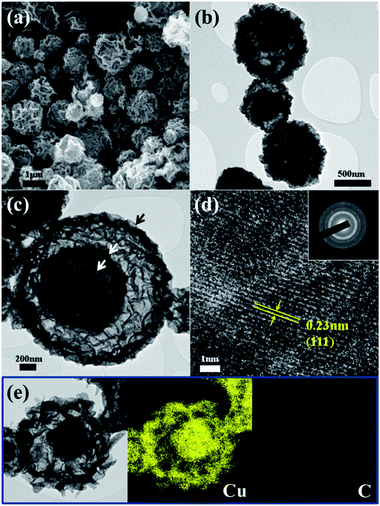 | ||
| Fig. 3 Morphologies and dot-mapping images of the powders post-treated at 300 °C: (a) SEM image, (b)–(d) TEM images, and (e) dot-mapping images. | ||
The electrochemical properties of the CuO yolk–shell powders post-treated at 300 °C were compared with those of the commercial CuO nanopowders (NPs) with a mean size of 50 nm, as shown in Fig. 4. Fig. 4a shows the discharge and charge curves of the CuO yolk–shell powders for the first 5 cycles at a constant current density of 500 mA g−1, in which the initial discharge curve exhibits three distinct voltage plateaus near 1.28, 1.15, and 0.85 V. This indicates that the multi-step electrochemical reduction of CuO into Cu and Li2O occurred through a Cu2O intermediate.32 The discharge capacities of the yolk–shell powders for the 1st and 2nd cycles were 1172 and 538 mA h g−1, respectively. The irreversible capacity loss, which is a common feature of the transition metal oxides, was high as 636 mA h g−1 for the first cycle, due to the irreversible reactions of electrolyte decomposition and the formation of solid electrolyte interphase (SEI) films.33 However, the discharge capacities increased from 538 to 635 mA h g−1 when the cycling number was increased from 2 to 20. The cyclic voltammograms (CVs), measured at a scan rate of 0.1 mV s−1, also revealed an increase in capacity following the second cycle, as shown in Fig. 4b by arrows. Although some of the CuO was reduced to metallic Cu during the formation of the CuO yolk–shell powders, the crystallite size of Cu was so small that it was undetectable in the XRD pattern. Nonetheless, the step-by-step activation of metallic Cu from the surface during the first 20 cycles does increase the capacities of the CuO yolk–shell powders. A similar capacity increase was not observed in CVs and charge–discharge curves of the commercial CuO nanopowders, as shown in Fig. 4c and d, respectively. Fig. 4d shows the cycling performances of the CuO yolk–shell powders and the commercial CuO nanopowders at a constant current density of 500 mA g−1. The initial discharge capacity of the CuO yolk–shell powders is higher than that of the commercial CuO nanopowders, yet the commercial CuO nanopowders have a higher discharge capacity in the second cycle. Moreover, the initial Coulombic efficiencies of the CuO yolk–shell and the commercial CuO nanopowders for the first cycle were 46 and 65%, respectively; the low value for the CuO yolk–shell powders being attributable to their high contact area with the liquid electrolyte. The discharge capacities of the CuO yolk–shell powders increased from the 151 cycle up to 726 mA h g−1 after 500 cycles, due to the formation of polymeric gel-like film on the active material.33,34 Conversely, the discharge capacities of the commercial CuO nanopowders decreased steadily from the 5 cycle down to 302 mA h g−1 after 240 cycles. Fig. 4e shows the rate performance of the CuO yolk–shell powders, in which the current density is increased from 500 to 2500 mA g−1 in a step-by-step manner, and then restored to 500 mA g−1. For each step, 10 cycles were measured to evaluate the rate performance. The stable reversible discharge capacities of the CuO yolk–shell powders decreased from 560 to 470 mA h g−1 as the current density was increased from 500 to 2500 mA g−1, and the discharge capacity recovered to 644 mA h g−1 when the current density was restored to 500 mA g−1. The electrochemical properties of the CuO yolk–shell powders are compared with those of the powders with various morphologies reported in the previous literatures, and the results are summarized in Table S1.† The highly porous CuO nanorods prepared by precipitation method had a discharge capacity of 654 mA h g−1 at a current density of 300 mA g−1 after 200 cycles.25 The carbon-coated CuO hollow powders synthesized via spray pyrolysis had a discharge capacity of 750 mA h g−1 at a current density of 670 mA g−1 after 300 cycles.31 The long-term cycling performance of the nanostructured CuO materials has not been reported in the previous literatures. However, the CuO yolk–shell powders showed high discharge capacity of 615 mA h g−1 even after 1000 cycles at a high current density of 1000 mA g−1 as shown in Fig. S4.† The prepared CuO yolk–shell powders had superior electrochemical properties to those reported in the previous literatures.
The structural stabilities of the CuO yolk–shell powders and the commercial CuO nanopowders during cycling are proven by the electrochemical impedance spectroscopy (EIS) measurements shown in Fig. 5. These impedance measurements were all carried out at room temperature both before and after 500 cycles at a current density of 500 mA g−1. The resulting Nyquist plots of the electrodes comprise a semicircle in the medium frequency region, assigned to the charge-transfer resistance; and a line inclined at ∼45° to the real axis at low frequencies, which corresponds to the lithium diffusion process within the electrodes.35,36 These results show that the charge transfer resistance of the CuO yolk–shell powders is much higher than that of the commercial CuO nanopowders, with the high contact area between the active material and electrolyte being responsible for the high charge-transfer resistance of the CuO yolk–shell powders. However, the CuO yolk–shell powders have a lower charge transfer resistance than the commercial CuO nanopowders after cycling, as shown in Fig. 5b. Fig. 5c shows the relationship between the real part of the impedance spectra (Zre) and ω−1/2 (where ω is the angular frequency in the low frequency region, ω = 2πf) in the low-frequency region after 500 cycles. The lower slope (σ, Warburg impedance coefficient) of the real part of the impedance spectra (Zre) versus ω−1/2 for the CuO yolk–shell powders reveals a higher lithium-ion diffusion rate than commercial CuO nanopowders.35,36 This is attributable to the structural stability of the CuO yolk–shell powders during cycling, which lowers the charge transfer resistance and therefore improves the lithium ion diffusion rate.
Conclusions
A new formation mechanism for yolk–shell powders was proposed based on spray pyrolysis combined with a PVP additive. Using this, spherical composite powders of metal salt–PVP were prepared by a one-pot spray pyrolysis, with CuO selected as the target material for this study based on the difficulties associated with preparing it by any previously reported method. These powders resulted from the transformation of a copper nitrate–PVP composite coated with a copper nitrate hydroxide–carbon composite at a post-treatment temperature of 300 °C. The initial decomposition of the copper nitrate hydroxide–carbon composite layer formed an outer CuO shell, while the subsequent step-wise contraction and decomposition resulted in a final CuO yolk–shell powder. These prepared CuO yolk–shell powders were found to have a high structural stability and superior electrochemical properties compared with commercial CuO nanopowders.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (no. 2012R1A2A2A02046367).Notes and references
- J. Liu, H. Xia, D. Xue and L. Lu, J. Am. Chem. Soc., 2009, 131, 12086–12087 CrossRef CAS PubMed.
- J. Liu, Y. Zhou, J. Wang, Y. Pan and D. Xue, Chem. Commun., 2011, 47, 10380–10382 RSC.
- C. M. Sim, S. H. Choi and Y. C. Kang, Chem. Commun., 2013, 49, 5978–5980 RSC.
- Y. N. Ko, Y. C. Kang and S. B. Park, Nanoscale, 2013, 5, 8899–8903 RSC.
- N. Liu, H. Wu, M. T. McDowell, Y. Yao, C. Wang and Y. Cui, Nano Lett., 2012, 12, 3315–3321 CrossRef CAS PubMed.
- G. Q. Zhang, L. Yu, H. B. Wu, H. E. Hoster and X. W. Lou, Adv. Mater., 2012, 24, 4609–4613 CrossRef CAS PubMed.
- J. Liu, S. Z. Qiao, J. S. Chen, X. W. Lou, X. R. Xing and G. Q. Lu, Chem. Commun., 2011, 47, 12578–12591 RSC.
- X. Y. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604–5618 CAS.
- D. Deng and J. Y. Lee, Chem. Mater., 2008, 20, 1841–1846 CrossRef CAS.
- J. S. Chen, C. M. Li, W. W. Zhou, Q. Y. Yan, L. A. Archer and X. W. Lou, Nanoscale, 2009, 1, 280–285 RSC.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- Y. J. Hong, M. Y. Son and Y. C. Kang, Adv. Mater., 2013, 25, 2279–2283 CrossRef CAS PubMed.
- M. Y. Son, Y. J. Hong and Y. C. Kang, Chem. Commun., 2013, 49, 5678–5680 RSC.
- S. H. Choi and Y. C. Kang, ChemSusChem, 2013, 6, 2111–2116 CrossRef CAS PubMed.
- S. H. Choi, Y. J. Hong and Y. C. Kang, Nanoscale, 2013, 5, 7867–7871 RSC.
- J. H. Ju and K. S. Ryu, J. Electrochem. Soc., 2011, 158, A814–A817 CrossRef CAS PubMed.
- F. Cheng, J. Liang, Z. Tao and J. Chen, Adv. Mater., 2011, 23, 1695–1715 CrossRef CAS PubMed.
- B. Wang, X. L. Wu, C. Y. Shu, Y. G. Guo and C. R. Wang, J. Mater. Chem., 2010, 20, 10661–10664 RSC.
- S. W. Ko, J. I. Lee, H. S. Yang, S. J. Park and U. Y. Jeong, Adv. Mater., 2012, 24, 4451–4456 CrossRef CAS PubMed.
- M. A. Dar, S. H. Nam, Y. S. Kim and W. B. Kim, J. Solid State Electrochem., 2010, 14, 1719–1726 CrossRef CAS.
- M. Xu, F. Wang, B. Ding, X. Song and J. Fang, RSC Adv., 2012, 2, 2240–2243 RSC.
- Q. Zhang, D. Xu, X. Zhou, X. Wu and K. Zhang, Small, 2014, 10, 935–943 CrossRef CAS PubMed.
- R. Wu, X. Qian, F. Yu, H. Liu, K. Zhou, J. Wei and Y. Huang, J. Mater. Chem. A, 2013, 1, 11126–11129 CAS.
- X. Guan, L. Li, G. Li, Z. Fu, J. Zheng and T. Yan, J. Alloys Compd., 2011, 509, 3367–3374 CrossRef CAS PubMed.
- L. Wang, H. Gong, C. Wang, D. Wang, K. Tang and Y. Qianab, Nanoscale, 2012, 4, 6850–6855 RSC.
- Q. Yu, H. Huang, R. Chen, P. Wang, H. S. Yang, M. X. Gao, X. S. Peng and Z. Yea, Nanoscale, 2012, 4, 2613–2620 RSC.
- C. S. Choi, Y.-U. Park, H. Kim, N. R. Kim, K. Kang and H. M. Lee, Electrochim. Acta, 2012, 70, 98–104 CrossRef CAS PubMed.
- L. Wang, W. Cheng, H. Gong, C. Wang, D. Wang, K. Tang and Y. Qian, J. Mater. Chem., 2012, 22, 11297–11302 RSC.
- R. Sahay, P. S. Kumar, V. Aravindan, J. Sundaramurthy, W. C. Ling, S. G. Mhaisalkar, S. Ramakrishna and S. Madhavi, J. Phys. Chem. C, 2012, 116, 18087–18092 CAS.
- X. Wang, D. M. Tang, H. Li, W. Yi, T. Zhai, Y. Bando and D. Golberg, Chem. Commun., 2012, 48, 4812–4814 RSC.
- Y. Xu, G. Jian, M. R. Zachariah and C. Wang, J. Mater. Chem. A, 2013, 1, 15486–15490 CAS.
- S. Grugeon, S. Laruelle, R. Herrera-Urbina, L. Dupont, P. Poizot and J. M. Tarascon, J. Electrochem. Soc., 2001, 148, A285–A292 CrossRef CAS PubMed.
- S. Laruelle, S. Grugeon, P. Poizot, M. Dolle, L. Dupont and J. M. Tarascon, J. Electrochem. Soc., 2002, 149, A627–A634 CrossRef CAS PubMed.
- J. Maier, Nat. Mater., 2005, 4, 805–815 CrossRef CAS.
- M. S. Park, Y. M. Kang, G. X. Wang, S. X. Dou and H. K. Liu, Adv. Funct. Mater., 2008, 18, 455–461 CrossRef CAS.
- X. Chen, N. Zhang and K. Sun, J. Mater. Chem., 2012, 22, 13637–13642 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06054g |
| This journal is © The Royal Society of Chemistry 2014 |




