Density functional study on the effect of a new ladder-type structure with different substituent groups (R = H, CH3, OCH3 and CN) for donor–acceptor copolymers†
Zhiyong Fu,
Wei Shen,
Rongxing He,
Xiaorui Liu,
Pei Yu,
Xian Peng and
Ming Li*
College of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, China. E-mail: liming@swu.edu.cn
First published on 7th August 2014
Abstract
A series of ladder-type fused-ring D–A (donor–acceptor) copolymers have been designed and synthesized as electron donors for BHJ photovoltaic cells, in previous work, and almost all of them focus on electron-rich units. The aim of this paper is to explore the effect of a new ladder-type multifused electron-deficient unit (DCDTBT) with different substituent groups (R = H, CH3, OCH3 and CN) on the ground state structure, electronic, optical and charge transfer properties of D–A copolymers for the improvements of photovoltaic performance. Based on the reported D–A copolymer (P1) which was composed of an electron-rich benzo[1,2-b:4,5-b′]dithiophene unit (BDT) and an electron-deficient 4,7-di(4-hexyl-2-thienyl)-2,1,3-benzothiadiazole unit (4DTBT), the 4DTBT unit was replaced with the ladder-type di[4,4-dicyclopenta]dithienylbenzothiadiazole (DCDTBT) unit to form a new copolymer (P2). The calculated results reveal that P2 has better planarity, wider and stronger optical absorption, and smaller reorganization energy than P1. Then the substituent groups (CH3, OCH3 and CN) were introduced at the bridging carbons of the DCDTBT unit to design seven new donor copolymers (P2a–P2c, P2a′–P2c′ and P3). From the calculated results, the introduction of the substituent groups into the DCDTBT unit can markedly improve the electronic, optical and charge transfer properties of those copolymers, but have a little influence on molecular backbone planarity. In particular, the introduction of the cyano-group into the DCDTBT unit can obviously reduce the HOMO/LUMO levels of the copolymers. Interestingly, the copolymers (P2c/P2c′) combining the cyano-group and methyl or methoxyl into the same bridging carbon of DCDTBT unit have excellent electronic, optical properties and charge transfer ability, as promising donor polymers for high-efficiency (∼7% for P2c, ∼9% for P2c′) organic solar cells. In this work, a design strategy is used to select a suitable ladder-type electron-deficient unit for further improving the photovoltaic performance of donors in solar cells.
1. Introduction
Bulk heterojunction (BHJ) polymer solar cells (PSCs) have been becoming greatly attractive due to their numerous potential advantages over conventional silicon-based solar cells, such as low cost, lightweight, flexibility and solubility to fabricate flexible large-area devices.1–3 In general, a donor and an acceptor segment constitute the active layers in organic polymer solar cells, assembled either in the form of a blend or in a bilayer structure.4 Fullerene and its derivates are generally as acceptor materials for organic solar cells.5,6 In most cases, people mainly study electron donors in the organic solar cells. Now, the D–A copolymers have been widely used as donor polymers with narrow band gaps in PSCs.7–10It is worth mentioning that the main strategies to improve the efficiency of PSCs as follows: (1) reduce the HOMO energy level of a donor molecule to obtain a large open circuit voltage (Voc), owing to the energy difference between the HOMO of the donor and the LUMO of the acceptor in direct proportion to the Voc,3 (2) decrease the band gap of a donor molecule to increase the short-circuit current (Jsc),11 (3) keep the LUMO level of a donor to be at least 0.3 eV higher than the LUMO level of the acceptor (fullerene derivatives) to guarantee the efficient charge separation at the donor–acceptor interface and overcome the binding energy of the intrachain exciton.12–14 Besides, the solubility and morphology of photovoltaic materials significantly influence the efficiency in the fabrication of the bulk heterojunction PSCs.
Di-2-thienyl-2′,1′,3′-benzothiadiazole (DTBT) is extensively used as an efficient electron-deficient (acceptor) unit in high-efficiency BHJ polymer solar cells, because it is convenient to tune the energy levels so as to adjust the absorption spectra.15,16 In this paper, the D–A copolymer15 P1 (Fig. 1) that is composed of 4DTBT acceptor unit and donor unit of BDT has been designed and synthesized for PSCs. However, P1 possesses a relatively large energy gap (1.98 eV) and high-lying HOMO level, which results in low short-circuit current (Jsc) and small Voc, respectively.
Considerable researches have demonstrated that the forced planarization by covalently fastening adjacent aromatic units by a carbon bridge in the electron-rich (donor) units of the polymer backbone includes the following advantages:17–22 (1) providing an effective way to strengthen the π conjugation and decrease the band gap (broad light absorption spectrum), (2) suppressing the rotational disorder around interannular single bonds to lower the reorganization energy, enhancing charge-carrier mobility and intermolecular hopping, (3) facilitating to introduce different substituent groups at the bridging carbons to adjust the orbital energy levels (such as reducing HOMO/LUMO levels). For instance, Wu and his co-workers20 developed two ladder-type multifused copolymers poly(fluorence-dicyclopentathiophene-alt-benzothiadiazole) (PFDCTBT) and poly(carbazole-dicyclopentathiophene-alt-benzothiadiazole) (PCDCTBT) where the 3-positions of two outer thiophenes are covalently tied with 3,6-positions of central fluorene or carbazole cores by a carbon bridge forming a rigid structure. The rigid structures of PFDCTBT and PCDCTBT possess strong π conjugation, narrow band gaps and high hole mobilities. As expected, the copolymers of PFDCTBT and PCDCTBT both show relatively good performance (2.8% and 3.7%). However, up to now, most of the studies have been focusing on electron-rich units rather than electron-deficient units in D–A donor polymers.
Questions may be raised when a ladder-type electron-deficient unit with different substituent groups (R = H, CH3, OCH3 and CN) are introduced in polymer backbone as follows: (1) How do the substituent groups affect the ground state geometries of these copolymers? (2) What is the effect of the substituent groups on the electronic, optical and charge transfer properties of these copolymers as well as the photovoltaic performances? (3) How to make the copolymers possess both low-lying HOMO energy levels and narrow band gaps by introducing the substituents at the bridging carbon of the polymer backbones? In order to elaborate these problems, we devise a ladder-type multifused acceptor unit (DCDTBT) to replace 4DTBT unit on the basis of the concept of Wu and his co-workers. In the unique DCDTBT unit (R = H), all the 3-positions of two outer thiophenes were covalently tied with the 3,3′-position of BT core by a carbon bridge, forming two cyclopentadienyl rings embedded in a rigid pentacyclic structure. Moreover, the substituents (CH3, OCH3 and CN) were introduced on the bridging carbon of the DCDTBT unit to adjust the ground state structure, electronic, optical and charge transfer properties of D–A copolymers for the improvements of photovoltaic performance. In this paper, this study presents us with a structural guideline to select a ladder-type multifused acceptor unit for further improving the photovoltaic performance of BHJSCs.
2. Computational details
All the DFT and TD-DFT calculations were performed with the aid of the Gaussian 09 program.23 Density functional theory (DFT) was used to optimize the neutral, cationic and anionic geometries of the oligomers. For the sake of simplifying the calculation, all the alkyl and alkoxy branched chains (Fig. 1) were replaced by methyl and methoxyl groups, respectively, while hydrogen atoms saturate the terminal groups of the repetitive units.24 To find a appropriate calculated method, P1 was calculated at three famous functionals (B3LYP,25,26 PBE0 and BHandHLYP27) with 6-31G**and 6-311G** basis sets (on the monomer and dimer models) to predict the HOMO energy level. The PBE0 method is well suited for sulfur-bearing molecules by Jacquemin's reports.28,29 The related theoretical investigation of P1 has been reported by Zhang's team.30The calculated results of the HOMO energy levels of M1 (monomer) and D1 (dimer) at B3LYP, PBE0 and BHandHLYP methods with 6-311G** level are listed in Table 1, respectively. By contrast, the calculated HOMO energy levels at the same methods with 6-31G** level for monomer and dimer models are listed in Table S1 (see ESI†). The calculated HOMO energy level was taken from the energy eigenvalue corresponding to the highest occupied (Kohn–Sham) KS orbital on the basis of many papers supporting the physical significance of the highest occupied KS HOMO energy eigenvalue.31,32
| Method | Oligomer | HOMO | ΔELU–HO | λ | Eg,TD | HOMOa | λa |
|---|---|---|---|---|---|---|---|
| a Experimental values (from ref. 15) “M” and “D” denote monomer and dimer, respectively. | |||||||
| PBE0 | M1 | −5.40 | 2.67 | 563 | 2.20 | −5.40 | 625 |
| 379 | 425 | ||||||
| D1 | −5.24 | 2.40 | 636 | 1.95 | |||
| 420 | |||||||
| B3LYP | M1 | −5.24 | 2.37 | 602 | 2.06 | ||
| 397 | |||||||
| D1 | −5.09 | 2.13 | 682 | 1.82 | |||
| 445 | |||||||
| BHand-HLYP | M1 | −6.13 | 4.30 | 443 | 2.80 | ||
| 311 | |||||||
| D1 | −5.96 | 4.02 | 476 | 2.61 | |||
| 326 | |||||||
Two ways have been used to calculate the energy gaps (band gaps) of the copolymer (P1). The first way is to take the difference between HOMO and LUMO energies which is calculated by DFT (ΔELU–HO in Table 1 and S1†). Another way is to directly calculate the S0 → S1 electronic transition energy with TDDFT (Eg,TD; in Table 1 and S1†). Likewise, the calculated results with a smaller basis set (B3LYP/6-31G**, PBE0/6-31G** and BHandHLYP/6-31G**) are listed in the Table S1† for comparison with 6-311G**. The calculated band gap with PBE0 functional (dimer model) at the 6-311G** basis set in the TDDFT approach is in excellent agreement with the experimental value of the copolymer (P1). Furthermore, in order to expound the variation of the HOMO levels and energy gaps with increasing conjugation lengths, P1 was calculated at PBE0/6-311G** level on oligomer models (monomer, dimer and trimer) for reducing the calculated cost. As shown in Table S3 (see ESI†), from monomer to trimer, the HOMO levels are gradually increased while the energy gaps are gradually decreased. Moreover, the related investigation has been reported by Zhang's team.30
Compared with the above-mentioned calculated results, they show that PBE0 method with 6-311G** basis set on dimer model is more appropriate for this article. The LUMO level was equal to the HOMO level plus to the TDDFT transition energy (ELUMO = EHOMO + Eg,TD) instead of taking from the unreliable KS LUMO eigenvalue.24,33,34 In order to reduce the error, the reorganization energies for hole (λh) and electron (λe) of the copolymers were predicted on the dimer model at the PBE0/6-311G** level. In this work, the ground state geometries were calculated by the spin-restricted DFT. Nevertheless, the spin-unrestricted DFT was used to optimize the doublet ion state geometries of the anionic and cationic units. All the calculations of the studied copolymers were carried out only in the gas phase.
3. Results and discussion
3.1. The ground state structural properties
The main optimized ground state structural parameters of P1–P3, P2a–P2c and P2a′–P2c′ are shown in Table 2. The optimized ground state structures of all copolymers are listed in Fig. S1.† As shown in Table 2, the calculated dihedral angles (C1–C2–C3–S1/S1–C4–C5–C6/C7–C8–C9–S2/S2–C16–C17–C18/S3–C19–C20–C21/C22–C23–C24–C25/C26–C27–C28–C29) of P2 are less than the corresponding dihedral angles of P1, meaning that the molecular backbone of P2 has better planarity than that of P1. Herein, P2 possesses a good planar molecular structure due to the following reasons: (1) the two thiophene-rings have alkyl branched-chains in the 4DTBT unit, whereas there is no alkyl branched-chains in the DCDTBT unit; (2) the two outer thiophenes of the DCDTBT unit are covalently tied with the 3,3′-position of BT core by a carbon bridge, forming a rigid pentacyclic structure. Due to the introduction of the DCDTBT electron-deficient unit in P2, it leads to the stronger conjugation effect and the shorter bond lengths (C2–C3/C4–C5/C8–C9/C16–C17/C19–C20/C23–C24/C27–C28) compared with P1.| P1 | P2 | P2a | P2b | P2c | P2a′ | P2b′ | P2c′ | P3 | |
|---|---|---|---|---|---|---|---|---|---|
| Bond length (Å) | |||||||||
| C2–C3 | 1.446 | 1.440 | 1.440 | 1.440 | 1.440 | 1.440 | 1.440 | 1.440 | 1.440 |
| C4–C5 | 1.449 | 1.434 | 1.430 | 1.431 | 1.430 | 1.442 | 1.436 | 1.437 | 1.431 |
| C8–C9 | 1.449 | 1.434 | 1.430 | 1.429 | 1.430 | 1.442 | 1.442 | 1.437 | 1.431 |
| C16–C17 | 1.445 | 1.439 | 1.439 | 1.439 | 1.440 | 1.439 | 1.439 | 1.440 | 1.440 |
| C19–C20 | 1.445 | 1.439 | 1.439 | 1.440 | 1.440 | 1.439 | 1.440 | 1.440 | 1.440 |
| C23–C24 | 1.449 | 1.435 | 1.431 | 1.432 | 1.431 | 1.443 | 1.437 | 1.438 | 1.432 |
| C27–C28 | 1.452 | 1.438 | 1.434 | 1.434 | 1.435 | 1.446 | 1.446 | 1.442 | 1.436 |
| Dihedral angle (°) | |||||||||
| C1–C2–C3–S1 | −32.17 | −15.39 | −16.92 | 11.29 | −13.19 | −11.95 | −12.24 | 15.67 | −13.44 |
| S1–C4–C5–C6 | 6.57 | 0.38 | 3.24 | 2.87 | −0.83 | 2.93 | 2.46 | −0.28 | −1.61 |
| C7–C8–C9–S2 | 7.33 | 0.36 | 2.37 | 0.17 | −1.71 | 2.48 | 0.32 | −0.46 | −2.62 |
| S2–C16–C17–C18 | −31.67 | −13.89 | 12.73 | 10.63 | 16.18 | −12.53 | −13.63 | 15.43 | 17.40 |
| S3–C19–C20–C21 | 27.89 | 14.49 | 10.75 | −16.21 | −12.64 | 10.18 | 12.80 | −16.39 | −14.98 |
| C22–C23–C24–C25 | −7.93 | 0.03 | 1.86 | 1.37 | −1.03 | 1.78 | 0.89 | 0.26 | 1.26 |
| C26–C27–C28–C29 | −5.71 | 0 | 1.80 | 1.09 | −1.08 | 1.86 | 0.71 | 0.27 | 1.25 |
For DCDTBT-based derivatives (P2, P2a–P2c, P2a′–P2c′ and P3), introducing different substituents (R = H, CH3, OCH3 and CN) to bridge-carbon only leads a slight geometrical parameter change. For example, compared with P2, the bond lengths (C2–C3/C16–C17/C19–C20) and the dihedral angles (C1–C2–C3–S1/S2–C16–C17–C18/S3–C19–C20–C21) of those copolymers (P2a–P2c, P2a′–P2c′ and P3) have a little variation. It indicates that the interaction between donor units (D) and acceptor units (A) is weakly influenced no matter what or how many R parts are introduced at bridging carbons of the DCDTBT electron-deficient unit. Specifically, for the all oligomers (except P1), the dihedral angles of S1–C4–C5–C6/C7–C8–C9–S2/C22–C23–C24–C25/C26–C27–C28–C29 are close to 0.0° due to the forced planarization by covalently fastening adjacent aromatic units by a carbon bridge. Therefore, the introducing of the different substituent groups into the DCDTBT unit has a little effect on the molecular conjugation effect and planarity.
3.2. Frontier molecular orbitals
We calculated the HOMO/LUMO energy levels of all the copolymers on a dimer model by DFT at the PBE0/6-311G** level. The orbital diagrams of HOMO and LUMO of all dimers are plotted in Fig. 2.For all of the copolymers, the HOMOs are π-type orbitals which are delocalized over the entire C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C backbone (with a little contribution of the C
C backbone (with a little contribution of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N–S bonds of the BT unit). But the LUMOs are almost completely focused on the electron-deficient moiety with π* character, and the contribution of thiazole ring (C
N and N–S bonds of the BT unit). But the LUMOs are almost completely focused on the electron-deficient moiety with π* character, and the contribution of thiazole ring (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N–S bonds) is markedly increased in LUMOs. The HOMO → LUMO electron transitions (see Fig. 2 and Table 4) in these copolymers imply the electron transfer from the electron-rich segment to electron-deficient segment. Furthermore, the prerequisite of an effective intramolecular charge transfer is that the HOMO and LUMO plots exhibit certain overlap.
N and N–S bonds) is markedly increased in LUMOs. The HOMO → LUMO electron transitions (see Fig. 2 and Table 4) in these copolymers imply the electron transfer from the electron-rich segment to electron-deficient segment. Furthermore, the prerequisite of an effective intramolecular charge transfer is that the HOMO and LUMO plots exhibit certain overlap.
In the Table 3, the values of molecular orbital energies (HOMO and LUMO, ELUMO = EHOMO + Eg,TD) and compositions were calculated by DFT for all the copolymers. As shown in Table 3, compared with P1, the HOMO and LUMO energies of P2 are increased. Accordingly, the compositions of the donor fragments in HOMOs and the acceptor fragments in LUMOs decrease from P1 to P2. For instance, in HOMOs, the electron-rich fragments account for 46.7% and 39.2% in P1 and P2, respectively. In LUMOs, the electron-deficient fragments take up 90.4% and 86.8% in P1 and P2, respectively. These orders imply that the energies of HOMO (LUMO) may have a negative correlation with the compositions of the electron-rich fragments (the electron-deficient fragments) in HOMOs (LUMOs). This may be helpful to understand that P2 possesses high-lying HOMO and LUMO energy levels in comparison with P1.
| Oligomers | Orbital | Energy | Donor | Acceptor |
|---|---|---|---|---|
| P1 | HOMO | −5.24 | 46.7 | 53.3 |
| LUMO | −3.29 | 9.6 | 90.4 | |
| P2 | HOMO | −5.08 | 39.2 | 60.8 |
| LUMO | −3.15 | 13.2 | 86.8 | |
| P2a | HOMO | −5.02 | 37.4 | 62.6 |
| LUMO | −3.18 | 13.1 | 86.9 | |
| P2b | HOMO | −5.24 | 41.4 | 58.6 |
| LUMO | −3.42 | 11.8 | 88.2 | |
| P2c | HOMO | −5.46 | 47.2 | 52.8 |
| LUMO | −3.66 | 10.3 | 89.7 | |
| P2a′ | HOMO | −5.23 | 41.9 | 58.1 |
| LUMO | −3.52 | 9.8 | 90.2 | |
| P2b′ | HOMO | −5.39 | 45.4 | 54.6 |
| LUMO | −3.68 | 9.2 | 90.8 | |
| P2c′ | HOMO | −5.54 | 50.3 | 49.7 |
| LUMO | −3.84 | 8.5 | 91.5 | |
| P3 | HOMO | −5.80 | 62.4 | 37.6 |
| LUMO | −4.10 | 7.9 | 92.1 |
Based on the above discussion, we come to analyze those copolymers (P2, P2a, P2a′ and P3) which introduce two same substituent groups in the bridging carbons of DCDTBT unit. As shown in Table 3, for P2, P2a, P2a′ and P3, which the substituent groups are H, methyl, methoxyl and cyano-group, the HOMO energies are −5.08, −5.02, −5.23 and −5.80 eV, with the order of P2a > P2 > P2a′ > P3. The compositions of donor fragments in HOMOs are in the order P2a < P2 < P2a′ < P3. It implies that the energies of HOMO have a negative correlation with the compositions of the donor fragments in HOMOs. The sequence of LUMO energies for P2, P2a, P2a′ and P3 is P2 ≈ P2a > P2a′ > P3, the order of their compositions of the electron-deficient fragments in LUMOs is P2 ≈ P2a < P2a′ < P3. Likewise, the orders imply that there is a negative correlation between LUMO energies and the compositions of the electron-deficient fragments in LUMOs. These results manifest that the variations of the molecular orbital energies and compositions for P2, P2a, P2a′ and P3 are primarily influenced by electron-withdrawing strength of the substituent groups. In this paper, the comparison of the electron-withdrawing strength of the substituent groups is via the calculated NBO charge analysis of the corresponding electron-deficient units in Table S2.†
Furthermore, the study for the number of cyano-groups can also certify the above result. As shown in Table 3, from P2a to P2c, as the number of cyano-groups at the bridging carbon increases, the energies of HOMO and LUMO are gradually decreased. There is an increased trend for the compositions of the donor fragments in HOMOs and the acceptor fragments in LUMOs. Similarly, it implies that the energies of HOMO and LUMO have a negative correlation with the compositions of the donor fragments in HOMOs and the acceptor fragments in LUMOs. The results demonstrate again that the variations of molecular orbital energies and compositions for the copolymers (P2a–P2c and P2a′–P2c′) are mainly influenced by electron-withdrawing strength of the substituent groups.
3.3. Absorption spectra
The simulated optical adsorption spectra and vertical singlet–singlet electronic transition energies of all the copolymers were computed with TD-PBE0/6-311/G**//PBE0/6-311G** level on a dimer model. The calculated dipole-allowed absorptions associated with electronic transitions, oscillator strength (f), and dominating configurations are summarized in Table 4 (considering the first 20 excited states). The simulated absorption spectra of the nine copolymers are presented in Fig. 3. In Fig. 3, there are two obvious absorption peaks of these copolymers in the visible and near-infrared regions. The strong transitions (f > 0.8) of all copolymers in the visible range correspond to the transitions from HOMO to LUMO, HOMO to LUMO+2 and HOMO−4 to LUMO. Fig. 2 and S2† show that the electronic transitions in these copolymers are assigned to π–π* type transition.| Excitation energy (eV and nm) | Transition | Oscillator | Main configuration | λmax,exp (nm) | ||
|---|---|---|---|---|---|---|
| a From ref. 15. | ||||||
| P1 | 1.95 | 636 | S0 → S1 | 1.6978 | H → L (92%) | 625a |
| 2.95 | 420 | S0 → S9 | 1.0376 | H → L+2 (85%) | 425a | |
| P2 | 1.92 | 644 | S0 → S1 | 2.0861 | H → L (92%) | |
| 2.76 | 450 | S0 → S6 | 1.7026 | H → L+2 (80%) | ||
| P2a | 1.84 | 673 | S0 → S1 | 2.0183 | H → L (91%) | |
| 2.71 | 458 | S0 → S6 | 1.6069 | H → L+2 (78%) | ||
| P2b | 1.82 | 682 | S0 → S1 | 1.9161 | H → L (93%) | |
| 2.74 | 452 | S0 → S8 | 1.6552 | H → L+2 (86%) | ||
| P2c | 1.80 | 690 | S0 → S1 | 1.8796 | H → L (92%) | |
| 2.77 | 448 | S0 → S9 | 1.5821 | H → L+2 (84%) | ||
| P2a′ | 1.71 | 726 | S0 → S1 | 1.6889 | H → L (92%) | |
| 2.72 | 456 | S0 → S8 | 1.7431 | H → L+2 (88%) | ||
| P2b′ | 1.71 | 726 | S0 → S1 | 1.6796 | H → L (92%) | |
| 2.74 | 452 | S0 → S9 | 1.6330 | H → L+2 (85%) | ||
| P2c′ | 1.70 | 728 | S0 → S1 | 1.6623 | H → L (91%) | |
| 2.76 | 449 | S0 → S9 | 0.8322 | H → L+2 (48%) H−4 →L (39%) | ||
| 2.78 | 446 | S0 → S10 | 0.8985 | H → L+2 (39%) H−4 →L (42%) | ||
| P3 | 1.70 | 729 | S0 → S1 | 1.6557 | H → L (82%) | |
| 2.79 | 445 | S0 → S10 | 1.5089 | H → L+2 (73%) | ||
As shown in Table 1, the calculated absorption peaks (P1: 636, 420 nm) reproduce well (within 11 nm of the error in the absorption peak position) the experiment absorption peaks15 (P1: 625, 425 nm). In Fig. 3a, compared with that of P1, P2 which is introduced ladder-type DCDTBT unit in the polymer backbone possesses stronger and wider light absorption. The lowest transition absorption peak of P2 only shows red-shifted of 8 nm in comparison with P1. However, the lowest transition absorption peaks of P2a–P2c, P2a′–P2c′ and P3 show red-shifted of 29, 38, 46, 82, 82, 84 and 85 nm in comparison with P2 (see Table 4), respectively. Obviously, introducing the substituent groups (CH3, OCH3 and CN) at the bridging carbons of the DCDTBT unit can exhibit significantly red-shifted absorptions. In particular, compared to P1 and P2, the copolymers of P2a–P2c, P2a′–P2c′ and P3 have much broader absorption in the visible and infrared region, and their maximum absorption peaks are close to the wavelength of the maximum photon flux density of the solar spectrum (∼700 nm, ∼1.77 eV).18
Interestingly, for P2a–P2c and P2a′–P2c′, the lowest absorption transition energies are almost no change while raising the number of cyano-groups (n = 0, 1, 2 for P2a–P2c and P2a′–P2c′, respectively.). From Fig. 3 and Table 4, it can be observed that the energy gaps don't change no matter how many cyano-groups are introduced on the ladder-type structure. This may result from the fact that the HOMO and LUMO levels are reduced at the same extent for P2a–P2c and P2a′–P2c′. In addition, the electron density distributions of HOMO and LUMO for P2a–P2c and P2a′–P2c′ have similar character. This may be helpful for us to understand that they have the similar absorption transition energies and the shape of absorption peaks. Likewise, for P2, P2a–P2c, P2a′–P2c′ and P3, the second absorption peaks are also almost invariable, which are mainly caused by the electron excitation from HOMO to LUMO+2. As expected, the electron density distribution plots of HOMO and LUMO+2 also have similar character for the copolymers (P2, P2a–P2c, P2a′–P2c′ and P3) in Fig. 2 and S2.†
3.4. How do the designed copolymers possess both low-lying HOMO energy levels and narrow band gaps?
It is well known that, the HOMO/LUMO energy levels and the band gap of a donor material should be optimized to enhance the PCE of an organic solar cell. An “ideal” conjugated donor polymer should have an appropriate HOMO energy level (−5.4 eV) and a narrow band gap (1.5 eV).11 In fact, introducing the substituents into polymer backbone is often an effective way to adjust electronic and optical properties as well as improve the photovoltaic performance. In the present work, the introduction of methyl or methoxyl into the bridging carbons of DCDTBT-based copolymers (P2a and P2a′) display small band gaps and high-lying HOMO/LUMO levels while P3 possesses deep-lying HOMO/LUMO levels with strong electron-withdrawing group (CN) being introduced at the bridging carbon. In order to get a donor polymer with suitable HOMO/LUMO levels and narrow band gap, we tried to combine the cyano-group and methyl or methoxyl into the bridging carbons of the DCDTBT unit. It is interesting to note that the copolymers (P2c, P2b′ and P2c′) possess low band gaps and appropriate HOMO energy levels while the methyl or methoxyl and cyano-group both are introduced at the DCDTBT unit.3.5. Charge transfer properties
In order to design a good candidate for the solar cell application, it is significantly important to comprehend the relationship between the charge transport property and the molecular structure. We investigated the charge transport property through the reorganization energy. The inner reorganization energy (λint) results from the fast change of the molecular geometry (such as a charge is removed or added from a molecule), and the outer reorganization energy (λext) denotes variations in the surrounding medium owing to the polarization effects.35 When the medium contribution to the relaxation energy is neglected (such as in a thin film), the inner reorganization energies are much larger than their external counterparts (λext).36,37 Under this circumstance, the external reorganization energy could be ignored. Thus, we would mainly discuss about the λint in this paper. In this case, the hole reorganization energy (λh) and electron reorganization energy (λe) values can be calculated by eqn (1) and (2):36,38| λh = [E+0 − E+] + [E0+ − E0] | (1) |
| λe = [E−0 − E−] + [E0− − E0] | (2) |
In the two equations, E0 represents the energy of neutral segment in neutral geometry. E+(E−) is the energy of cation (anion) in cationic (anionic) geometry. E0+(E0−) denotes the energy of neutral segment in cationic (anionic) geometry, while E+0(E−0) represents the energy of cation (anion) in neutral geometry. The calculated λh and λe are shown in Table 5. Compared with the representative hole transport material N,N′-diphenyl-N,N′-bis(3-methlphenyl)-(1,10-biphenyl)-4,4′-diamine (TPD) (λh = 0.290 eV), the λh values of the investigated polymers on a dimer model (0.200–0.279 eV) are smaller, which implies that the investigated polymers may have higher hole transfer rates.39 Similarly, the λe values (0.119–0.180 eV) are smaller than that of the typical electron transport material tris(8-hydroxyquinolinato)aluminum(III) (Alq3) (λe = 0.276 eV), which indicates that the investigated polymers may have higher electron transfer rates.40
| P1 | P2 | P2a | P2b | P2c | P2a′ | P2b′ | P2c′ | P3 | |
|---|---|---|---|---|---|---|---|---|---|
| λh | 0.279 | 0.208 | 0.201 | 0.205 | 0.205 | 0.206 | 0.209 | 0.206 | 0.198 |
| λe | 0.180 | 0.141 | 0.138 | 0.131 | 0.126 | 0.148 | 0.142 | 0.135 | 0.119 |
For P2, the values of λh and λe both are smaller than that of P1. This result demonstrates that the ladder-type multifused acceptor unit (DCDTBT) can suppress the rotational disorder around interannular single bonds to lower the reorganization energy in comparison with the 4DTBT acceptor unit. Moreover, the difference between λh and λe values for DCDTBT-based derivative (P2) is smaller than that of 4DTBT-based derivative (P1). It indicates that P2 has better equilibrium property for hole and electron transport than P1.
For DCDTBT-based derivatives, their λh values are 0.208, 0.201, 0.205, 0.205, 0.206, 0.209, 0.206 and 0.198 eV for P2, P2a–P2c, P2a′–P2c′ and P3, respectively, and their λe values are 0.141, 0.138, 0.131, 0.126, 0.148, 0.142, 0.135 and 0.119 eV, respectively. Surprisingly, the λh values of those copolymers are very nearly close. These results suggest that the introduction of the different substituent groups (H, CH3, OCH3 and CN) at the bridging carbons of the ladder-type multifused DCDTBT unit has a large effect on λe but hardly influence λh. This may be due to the fact that these copolymers possess very similar ground state geometric structures. For the copolymers of P2a–P2c, their λh values are almost the same, and their λe values decrease in the following order: P2a > P2b > P2c. Likewise, the copolymers of P2a′–P2c′ also have the same tendency with P2a–P2c. The results reveal that the raising number (such as n = 0, 1, 2 for P2a–P2c, respectively.) of cyano-groups can decrease the λe values gradually but make little effect on λh.
3.6. Solar cell performances
In the polymer BHJ solar cells, two equally important parameters in determining the power conversion efficiency (PCE) of a given solar cell are short circuit current density (Jsc) and open circuit voltage (Voc). Moreover, a desirable polymer donor should exhibit not only a lowest transition energy between 1.2 and 1.9 eV with broad absorption to maximize sunlight absorption but also a highest occupied molecular orbital energy level between −5.2 and −5.8 eV.41 The Voc and Jsc are largely influenced by the HOMO energy levels and the band gaps of the conjugated polymers, respectively. As shown in Table 4, the band gaps of P2, P2a–P2c, P2a′–P2c′ and P3 all are lower than that of P1. It may suggest that these newly designed copolymers (P2, P2a–P2c, P2a′–P2c′ and P3) may have larger Jsc than P1. In general, the Voc is proportion to the built-in potential which is defined as the difference between the HOMO level of a donor polymer and the LUMO level of an acceptor ([6,6]-phenyl-C61-butyric acid methyl ester (PCBM)). Since 1995, PC61BM/PC71BM (PC71BM, [6,6]-phenyl-C71-butyric acid methyl ester) are widely used as the standard acceptor because of good solubility, high electron mobility and commercial availability in organic solar cells.42–45 In this study,15 PC61BM is selected as the electron acceptor with the HOMO and LUMO energy levels of −6.1 eV and −4.2 eV, respectively. We can estimate the open circuit voltage (Voc) value of a conjugated polymer–PCBM solar cell as eqn (1)46| Voc = 1/e(|EdonorHOMO| − |EPCBMLUMO|) − 0.3 V(3) |
 | ||
| Fig. 4 Frontier molecular orbital energies of P1–P2, P2a–P2c, P2a′–P2c′ and P3. (ELUMO = EHOMO + Eg,TD). | ||
In order to estimate the performance of the designed molecules from the qualitative perspective, we used the Scharber diagram46 to predict PCEs (%) of the solar cells of those eight donors. From Scharber diagram, the PCE of PSC can be predicted by the band gap and the LUMO level of the constituent donor polymer. Scharber et al. proposed the design rule, which suppose a fill factor (FF) of 0.65 and a carrier mobility of ∼10−3 cm2 V−1 S−1. For the sake of power conversion efficiencies exceeding 10%, a donor polymer should have a LUMO energy level < −3.92 eV and a band gap < 1.74 eV. Fig. 5 shows that the PCE is more sensitive to the LUMO level of donor polymer than the band gap. Now, many researches have revealed that the LUMO energy levels of D–A donor copolymers mainly rely on the electron-accepting moieties.47 We estimate the PCEs of P1 (using the calculated results of Eg,TD and ELUMO = EHOMO + Eg,TD) is ∼4.4% by the Scharber diagram, and it agrees well with the experimental data (5.0% ref. 15).
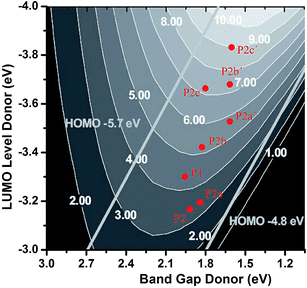 | ||
| Fig. 5 Scharber diagrams to estimate PCEs (%) of the bulk heterojunction solar cells of the copolymers (P1, P2, P2a–P2c and P2a′–P2c′). Adapted with permission from the work of Scharber and coworkers (in ref. 46). Copyright 2006 Wiley-VCH. | ||
According to the Scharber diagram, the corresponding PCEs of these copolymers are ∼3.5% (P2), ∼3.6% (P2a), ∼5% (P2b), ∼7% (P2c), ∼6% (P2a′), ∼7.5% (P2b′), and ∼9% (P2c′), respectively. Compared with that of P1, the results show that P2c, P2b′ and P2c′ have smaller band gaps and deeper LUMO energy levels, and exhibit the higher predicted PCEs (∼7%, ∼7.5% and ∼9% for P2c, P2b′ and P2c, respectively) when combined with PC61BM acceptor in organic solar cells. The results demonstrate that our idea is an efficient way to improve the performance of the D–A conjugated polymers.
4. Conclusion
We presented several polymer donors for elaborating how a ladder-type multifused acceptor unit with different substituent groups (R = H, CH3, OCH3 and CN) that are introduced in polymer backbone, affect the ground state structure, electronic, optical and charge transfer properties. First, the introduction of the ladder-type DCDTBT unit to replace 4DTBT unit in the donor not only enhances the π conjugation and the intrachain coplanarity in molecular backbone, but also decreases the band gap and reorganization energy. In addition, compared with the calculated results of P2, the introducing of the substituent groups into DCDTBT unit can further adjust electronic, optical and charge transport properties of the copolymers but with a little effect on molecular backbone planarity. In particular, the introduction of the strong electron-withdrawing cyano-group onto the bridging carbon of DCDTBT unit can evidently reduce HOMO and LUMO levels. Moreover, the calculation shows that P2c and P2c′ have not only a favorable electronic structure (deeper HOMO and LUMO levels) but also lower band gaps and reorganization energies in comparison with P1. Especially the predicted PCEs of these newly designed copolymers (P2c and P2c′) are ∼7% and ∼9%, respectively. Therefore, P2c and P2c′ may be promising donors for organic bulk heterojunction solar cells in the future. These results may provide a design idea for selecting a suitable ladder-type electron-deficient unit for further improving the performance of donors in the PSCs.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant no. 21073144), and by Fundamental Research Funds for the Central Universities (grant no. XDJK2010B009).References
- G. Yu, J. Gao, J. Hummelen, F. Wudl and A. Heeger, Science, 1995, 270, 1789–1790 CAS.
- R. Friend, R. Gymer, A. Holmes, J. Burroughes, R. Marks, C. Taliani, D. Bradley, D. Dos Santos, J. Bredas and M. Lögdlund, Nature, 1999, 397, 121–128 CrossRef CAS PubMed.
- H.-Y. Chen, J. Hou, S. Zhang, Y. Liang, G. Yang, Y. Yang, L. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649–653 CrossRef CAS.
- Y. Yi, V. Coropceanu and J.-L. Brédas, J. Am. Chem. Soc., 2009, 131, 15777–15783 CrossRef CAS PubMed.
- N. Sariciftci, L. Smilowitz, A. Heeger and F. Wudl, Science, 1992, 258, 1474–1476 CAS.
- H. Imahori, H. Yamada, D. M. Guldi, Y. Endo, A. Shimomura, S. Kundu, K. Yamada, T. Okada, Y. Sakata and S. Fukuzumi, Angew. Chem., 2002, 114, 2450–2453 CrossRef.
- J. Peet, J. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger and G. C. Bazan, Nat. Mater., 2007, 6, 497–500 CrossRef CAS PubMed.
- J. Hou, H.-Y. Chen, S. Zhang, G. Li and Y. Yang, J. Am. Chem. Soc., 2008, 130, 16144–16145 CrossRef CAS PubMed.
- Y. Chen, R. N. Gunasinghe, X.-Q. Wang and Y. Pang, RSC Adv., 2013, 3, 25097–25102 RSC.
- Y. Chen, Y. Xu, Q. Wang, R. N. Gunasinghe, X. Q. Wang and Y. Pang, Small, 2013, 9, 870–875 CrossRef CAS PubMed.
- B. C. Thompson and J. M. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed.
- Z. He, C. Zhong, X. Huang, W. Y. Wong, H. Wu, L. Chen, S. Su and Y. Cao, Adv. Mater., 2011, 23, 4636–4643 CrossRef CAS PubMed.
- N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. Neagu-Plesu, M. Belletête, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732–742 CrossRef CAS PubMed.
- G. Dennler, M. C. Scharber and C. J. Brabec, Adv. Mater., 2009, 21, 1323–1338 CrossRef CAS PubMed.
- H. Zhou, L. Yang, A. C. Stuart, S. C. Price, S. Liu and W. You, Angew. Chem., 2011, 123, 3051–3054 CrossRef PubMed.
- H. Zhou, L. Yang, S. Liu and W. You, Macromolecules, 2010, 43, 10390–10396 Search PubMed.
- L. Koster, V. Mihailetchi and P. Blom, Appl. Phys. Lett., 2006, 88, 093511–093513 Search PubMed.
- Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868–5923 CrossRef CAS PubMed.
- P. Coppo and M. L. Turner, J. Mater. Chem., 2005, 15, 1123–1133 Search PubMed.
- J.-S. Wu, Y.-J. Cheng, M. Dubosc, C.-H. Hsieh, C.-Y. Chang and C.-S. Hsu, Chem. Commun., 2010, 46, 3259–3261 RSC.
- C.-H. Chen, Y.-J. Cheng, C.-Y. Chang and C.-S. Hsu, Macromolecules, 2011, 44, 8415–8424 Search PubMed.
- R. N. Gunasinghe, D. G. Reuven, K. Suggs and X.-Q. Wang, J. Phys. Chem. Lett., 2012, 3, 3048–3052 CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, et al., Gaussian 09, Revision A.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- J. Ku, Y. Lansac and Y. H. Jang, J. Phys. Chem. C, 2011, 115, 21508–21516 CAS.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200–1211 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 1372 CrossRef CAS PubMed.
- D. Jacquemin and E. A. Perpète, Chem. Phys. Lett., 2006, 429, 147–152 CrossRef CAS PubMed.
- D. Jacquemin, J. Preat, V. Wathelet, M. Fontaine and E. A. Perpète, J. Am. Chem. Soc., 2006, 128, 2072–2083 CrossRef CAS PubMed.
- L. Zhang, K. Pei, M. Yu, Y. Huang, H. Zhao, M. Zeng, Y. Wang and J. Gao, J. Phys. Chem. C, 2012, 116, 26154–26161 CAS.
- J. P. Perdew and M. Levy, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 16021 CrossRef CAS.
- D. Chong, O. Gritsenko and E. Baerends, J. Chem. Phys., 2002, 116, 1760 CrossRef CAS PubMed.
- L. Yang, J.-K. Feng and A.-M. Ren, J. Mol. Struct.: THEOCHEM, 2007, 816, 161–170 CrossRef CAS PubMed.
- G. Zhang and C. B. Musgrave, J. Phys. Chem. A, 2007, 111, 1554–1561 CrossRef CAS PubMed.
- V. Lemaur, M. Steel, D. Beljonne, J.-L. Brédas and J. Cornil, J. Am. Chem. Soc., 2005, 127, 6077–6086 CrossRef CAS PubMed.
- S. Tang and J. Zhang, J. Phys. Chem. A, 2011, 115, 5184–5191 CrossRef CAS PubMed.
- N. G. Martinelli, J. Idé, R. S. Sánchez-Carrera, V. Coropceanu, J.-L. Brédas, L. Ducasse, F. Castet, J. Cornil and D. Beljonne, J. Phys. Chem. C, 2010, 114, 20678–20685 CAS.
- W.-Q. Deng and W. A. Goddard, J. Phys. Chem. B, 2004, 108, 8614–8621 CrossRef CAS.
- N. E. Gruhn, D. A. da Silva Filho, T. G. Bill, M. Malagoli, V. Coropceanu, A. Kahn and J.-L. Brédas, J. Am. Chem. Soc., 2002, 124, 7918–7919 CrossRef CAS PubMed.
- B. C. Lin, C. P. Cheng, Z.-Q. You and C.-P. Hsu, J. Am. Chem. Soc., 2005, 127, 66–67 CrossRef CAS PubMed.
- Y. Zou, A. Najari, P. Berrouard, S. Beaupré, B. Réda Aïch, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2010, 132, 5330–5331 CrossRef CAS PubMed.
- K.-H. Kim, H. Kang, S. Y. Nam, J. Jung, P. S. Kim, C.-H. Cho, C. Lee, S. C. Yoon and B. J. Kim, Chem. Mater., 2011, 23, 5090–5095 Search PubMed.
- C. Zhang, S. Chen, Z. Xiao, Q. Zuo and L. Ding, Org. Lett., 2012, 14, 1508–1511 CrossRef CAS PubMed.
- Y. Sun, G. C. Welch, W. L. Leong, C. J. Takacs, G. C. Bazan and A. J. Heeger, Nat. Mater., 2011, 11, 44–48 CrossRef PubMed.
- C. J. Brabec, C. Winder, N. S. Sariciftci, J. C. Hummelen, A. Dhanabalan, P. A. van Hal and R. A. Janssen, Adv. Funct. Mater., 2002, 12, 709–712 CrossRef CAS.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789–794 CrossRef CAS PubMed.
- H. Zhou, L. Yang, S. Stoneking and W. You, ACS Appl. Mater. Interfaces, 2010, 2, 1377–1383 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05954a |
| This journal is © The Royal Society of Chemistry 2014 |



