Two dimensional TiO2 nanosheets: in vivo toxicity investigation
Sha-Sha Song†
a,
Bao-Yu Xia†b,
Jie Chena,
Jiang Yangd,
Xiu Shena,
Sai-Jun Fana,
Mei-li Guo*c,
Yuan-Ming Sun*a and
Xiao-Dong Zhang*a
aTianjin Key Laboratory of Molecular Nuclear Medicine, Institute of Radiation Medicine, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China. E-mail: yuanmings1962@163.com; Xiaodongzhang@tju.edu.cn
bSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore
cDepartment of Physics, School of Science, Tianjin Chengjian University, Tianjin, China. E-mail: meiliguo314@gmail.com
dDepartment of Biological Systems Engineering, University of Wisconsin-Madison, Madison, WI, USA
First published on 3rd September 2014
Abstract
Titanium dioxide (TiO2) nanosheets have received attention for photodynamic therapy due to their unique electronic structure and high surface activity. However, the biological response and toxicity of two dimensional (2D) nanomaterials are still not clear. Herein, in vivo toxicity of TiO2 nanosheets, such as biodistribution, hematology, biochemistry and pathology, were evaluated at the dose of 10 mg kg−1 by intraperitoneal injection for up to 30 days. It was found that TiO2 could gradually accumulate in the liver and spleen with increase of exposure time, which is due to large size induced absorption from the reticuloendothelial system (RES). Furthermore, the hematological data indicated that no significant difference was found. However, the biochemistry showed that the liver indicator, AST, presented a significant difference after 30 days compared with control mice. The present work revealed that 2D TiO2 nanosheets did not cause appreciable toxicity, but induced accumulation in the liver and slight abnormality of the liver with increasing exposure time.
Introduction
Titanium dioxide (TiO2) has attracted lots of attention in different areas, such as cosmetics,1 the paint industry,2 pharmaceutics and biomedical applications,3 due to its high stability, anticorrosion properties4 and photocatalysis.5,6 Highly active TiO2 can be used for a photothermal therapy contrast agent.7 Meanwhile, TiO2 can show an effective bactericidal activity even under very weak UV light illumination, and its biological activity has been confirmed by in vivo experiment.8,9 Unfortunately, in vivo toxicity of TiO2 is still a big challenge, and it is necessary to understand their biological response.The in vivo biodistribution and toxicity of nanomaterials are closely related to the physical dimension and surface chemistry.10–18 In the past several years, several groups investigated the in vitro and in vivo experiments of TiO2 nanoparticles (NPs).19,20 Park, et al. showed that surface area of TiO2 nanowires may play an important role in biological response. They found that the increase of autophagosome-like vacuoles may be an important reason for cytotoxicity due to NPs induced reactive oxygen species (ROS).21 Warheit, et al. revealed that anatase TiO2 nanorods and nanodots did not lead to lung inflammation or pathological changes.22 Jin, et al. reported that spontaneous ROS was generated by anatase-TiO2 exposure and then could induce cellular apoptosis, but was not found in rutile-TiO2 NPs.23 Similarly, dose-dependent effects of TiO2 were widely investigated. Fabian, et al. reported that TiO2 with lower dose (5 mg kg−1) showed no damage to all of the organs.24 The biodistribution showed that the lots of Ti could be found in the liver, kidney, and spleen after injecting anatase TiO2 nanoparticles (5, 10, 50 mg kg−1). As a contrast, TiO2 with higher dose (100, 150 mg kg−1) caused serious damage to the liver, kidney, and myocardium of mice.25 A similar study showed injected dose of TiO2 performed the significant influence on toxicity of mice.26,27
Despite of lots of in vivo toxicity investigations, the biological response of two dimensional (2D) nanosheets is still less reported. Compared with traditional nanoparticles, nanorods, and nanowires, TiO2 nanosheets with high surface area can introduce many interesting chemical and biological properties.28,29 Therefore, it is necessary to investigate the biological effects of 2D TiO2 nanosheets. In this work, we present the in vivo toxicity of TiO2 nanosheets systematically. In vivo biodistribution, the immunogenicity, hematological toxicity, and damages in liver and spleen, were investigated in detail.
Materials and methods
Fabrication
The anatase TiO2 nanosheets were synthesized through a modified hydrothermal method.30–32 In a typical synthesis, 5 mL of titanate isopropoxide (97%, Sigma-Aldrich) was added into a 40 mL Teflon-lined autoclave. Then 0.6 mL of 48% HF solution was added drop-wise. The mixed solution was sealed and kept in an electric oven at 180 °C for 24 h. Then it was naturally cooled down to room temperature. After that, N-(carbonyl-methoxypolyethyleneglycol 5000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE-PEG5000) was used to coat the TiO2 nanosheets. Typically, 20 mg DSPE-PEG5000 was dispersed in 20 mL pure water, and then added the TiO2 nanosheets (1 mg) into the solution, and then washed 6 times.33 Finally, we got the DSPE-PEG5000 coated TiO2 nanosheets. Transmission electron microscope (TEM) analysis was conducted at 200 kV with JEOL models (JEM-2100F and JEM-2011HC).Animal administration
Animals were purchased, maintained, and handled with protocols approved by the Institute of Radiation Medicine, Chinese Academy of Medical Sciences (IRM, CAMS). The C57 mice, obtained from IRM laboratories at 8 weeks of age, were housed in a 12 h/12 h light/dark cycle. The temperature was maintained at 20 ± 2 °C, relative humidity at 60 ± 10%, based on the previously identified factor.34 Distilled water and sterilized food for mice were available ad libitum. Mice were acclimated to this environment for 3 days. Then, mice were randomly divided into five groups (seven in each group) for one control group and four experimental groups, respectively. The experimental mice received 200 μL of TiO2 solution at a dose of 1 mg mL−1. The mice were weighted and assessed for behaviour and symptom every other day for 30 days post-injection.Hematology, biochemistry and sample collection
Mice were sacrificed, and blood, serum and organs were collected for hematological, biochemical, immune, and histopathologic analysis after 1, 7, and 30 days post-injection. Using a standard blood collection technique, 20 μL blood was drawn from the saphenous vein into a potassium ethylenediamine tetra-acetic acid collection tube for hematological analysis. Mice were sacrificed using isoflurane anesthetic and exsanguinated with phosphate-buffered saline using an angiocatheter. Serum was harvested by centrifuging blood at 6000 rpm for 5 minutes. During necropsy, heart, liver, spleen, lung, kidney, thymus, and testis were collected. One mouse from each group was fixed with 10% buffered formalin following exsanguination. To explicitly examine the immune response, spleen and thymus indexes (Sx) can be defined as:Major organs from those mice were processed routinely into paraffin, and stained with hematoxylin and eosin (H&E). Pathology was examined using a digital microscope.35
Results and discussions
Characterization of 2D TiO2 nanosheet by TEM and SEM
The DSPE-PEG5000 coated TiO2 was fabricated by pervious reported methods.36–38 The characterization of size and morphology of TiO2 was presented by transmission electron microscope (TEM) and scanning electron microscope (SEM) in Fig. 1. It could be seen that DSPE-PEG5000 coated TiO2 nanosheets performed the uniform dispersion. Average size of TiO2 nanosheets was 92.5 nm measured by TEM and SEM. According to our pervious investigation, the phase of TiO2 nanosheets was anatase.30,31 Anatase TiO2 showed the very high photocatalytic activity and wide application in energy and environment, and toxicity of anatase TiO2 will also be interesting.39 We next move to in vivo toxicity of TiO2 nanosheets.Body weight and immune response
The body weight is an important indicator for evaluating the toxicological effects of the DSPE-PEG5000 coated TiO2. Fig. 2 showed the time-dependent variation of body weight of control and TiO2 treated mice in the period of 30 days. The body weight of mice was recorded every 2 days. No changes, such as vocalizations, labored breathing, were detected in each group. Difference of body weight between normal mice and treated mice was not found after 1–12 days injection. However, after injection of 14 days, the body weight of normal mice performed more obvious increase than that of TiO2 treated mice. Using Student's t-test, no significant statistical difference between normal mice and treated mice was found. The P values were 1.55 for 14 days and 0.179 for 30 days, respectively. The small weight difference between the two groups indicated that the TiO2 could lead to potential toxicity in mice. Similar trend was observed after injection 30 days.Fig. 3 presented the immune response of mice treated with TiO2 based on thymus and spleen indexes. We calculated the spleen and thymus indexes in order to examine immune system damage. For the spleen index, no significant statistical difference was detected between the control and the treated groups by using Student's t-test. Generally, the increase of spleen index indicated the immune response of mice, which had been widely reported elsewhere.40 It could be concluded that the spleen and thymus indexes of the treated mice had no significant changes, which indicated no obvious immune response was found.
The indexes of spleen and thymus are important indicators for immune system of mice.41 In spite of no significant statistical difference, the DSPE-PEG5000 coated TiO2 still caused the slight change of spleen index in treated mice. The change may be related to the preferable retention of TiO2 in the spleen. These changes inspire our interest to explore more findings, such as biodistribution, hematology, and biochemistry.
Biodistribution
The biodistribution of TiO2 treated mice was presented in Fig. 4. It was observed that 4.32% ID/g could be found in liver after 24 h, and then reduced to 4.03% ID/g after 7 days. After 30 days, the uptake of liver decreased to 1.21% ID/g and uptake of spleen sharply increased to 10.42% ID/g. The main reason is that spleen is the largest organ of the immune system.34 When exogenous nanoparticles enter in body, they will be firstly absorbed by reticuloendothelial system (RES) and thus induce the high distribution in the spleen which was consistent with previous results.25 The accumulation of TiO2 in spleen may come from gradual absorption of TiO2. Meanwhile, the decrease of uptake in liver indicated that partial TiO2 were excreted by the liver. Recently, our groups reported that Bi2Si3 NPs mostly was cleared from mice body by 90 days post-injection, and the residual amount was less than 5%.42 Meanwhile, PEG-coated gold nanoparticles preferred to stay in liver at an exposure time of 30 days.43 Compared with these results, 2D TiO2 nanosheets show long retention time.44Hematology and biochemistry
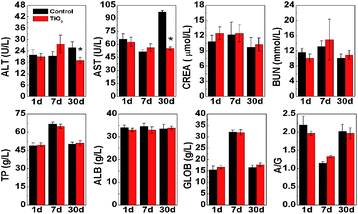
TiO2 will firstly interact with the blood components when they are injected into mice. Thus, it is very important to investigate the hematologic factors change, such as white blood cell (WBC).34 Fig. 5 performed the hematologic analysis of mice both control and treated groups. We analyzed standard hematologic markers, such as WBC, red blood cell (RBC), platelet (PLT), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). As is well known, WBC and RBC are the most important indicators for in vivo response, which are directly related to infection and inflammation. The WBC and RBC did not show significant changes and maintain a normal range, which indicated that TiO2 did not cause serious infections. PLT showed a slight increase after 7 days (P = 0.01). HTC showed an obvious decrease after 30 days (P = 0.001), and MCH and MCHC showed significant increase after 30 days (P = 0.001), which indicated that TiO2 induced slight damage in blood system. Other parameters, such as HGB and MCV, had no significant difference. Thus, it was clear that TiO2 nanosheets did not cause the significant infection but induce a slight toxic response after 30 days.It is necessary to investigate whether TiO2 cause toxicity in liver and kidney. Thus, we investigated the blood biochemistry. We presented the biochemical parameters, such as, alanine transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), creatinine (CREA), total protein (TP), albumin (ALB), globulin (GLOB), and albumin to globulin (A/G) in Fig. 6. We firstly focused on AST, ALT, and CREA because they are closely related to the function of liver and kidney, respectively. For the treated mice, the AST and ALT levels significantly decreased after 30 days, and statistical differences could be found compared with control group, and corresponding P values were 0.001 and 0.021, respectively. Meanwhile, CREA also showed slight increase, but no significant statistical difference could be found. No significant statistical difference was found for other indicators, such as BUN, ALB, TP, A/G, and GLOB. Thus, it could be concluded that injection of TiO2 caused the significant toxicity in liver after 30 days.45 It has been shown that TiO2 preferred to stay in liver and spleen by blood circulation. Furthermore, TiO2 could react with proteins in blood and further form the complexes of protein corona.46 Hybrid corona in the blood could accumulate in liver and cause the low liver damage.
Histopathology
To investigate toxicity, histological assessments were performed to determine whether TiO2 could cause tissue damage, inflammation or lesions. Finally, histological images of harvested organs, including heart, liver, spleen, lung, kidney and testicle, were performed to evaluate potential toxicity. It could be seen from Fig. 7 that no apparent damages and histopathological abnormalities were observed for the mice treated with TiO2. The pathological results provided macroscopic and visual evidence of toxicity. Appreciable pathological changes or organs damage were not found in the interested organs. | ||
| Fig. 7 Pathological images from the control and TiO2 injected mice at various time points post-injection. | ||
Oxidative stress response
To further confirm the damage of liver and lung, we performed the time dependent malondialdehyde (MDA) and superoxide dismutase (SOD). Oxidative stress response is related to the emergence of ROS.47 The content of MDA and SOD reflected the damaged level of organism resulting from free radicals. As seen from Fig. 8, the level of MDA decreased in liver, inversely, the SOD increased, which indicated that the clearance ability of free radicals was enhanced after 24 h. After 7 days, the SOD and MDA of liver and lung were recovered, which showed that the damaged level of free radicals was recovered. Statistical differences could be found for SOD and MDA after 24 h by using the Student's t-test, and corresponding P values were 0.0005 and 0.001. Therefore, injection of TiO2 could induce acute influence on ROS by decreasing level of MDA and increasing SOD after 24 h.From these results, we concluded that TiO2 could accumulate in liver, and thus induced liver toxicity. Furthermore, hematological and blood biochemical analysis presented significant changes in liver. Finally, the decreased level of MDA and increased SOD after 24 h indicated that active defense for free radical could be found. Therefore, we concluded that the accumulation of TiO2 induced liver toxicity at the present dose level.
These results suggested that further metabolism and toxicity of TiO2 should be investigated. Nanomaterials may generate both acute and chronic toxic effects. We speculate the toxicity of TiO2 is closely related to protein binding. In addition, some interactions, such as enhanced binding and conformational change of the proteins, may influence the in vivo toxicity.34 Furthermore, it is not clear whether TiO2 in the liver will cause abnormal gene expression. The change of key indicators, such as ALT, AST and CERA, are still not clear for a longer time. Thus, further study is necessary to preferably understand toxicity.
Conclusion
We completed systematic examinations of the potential toxicity of DSPE-PEG5000 coated TiO2 at different exposure times. The DSPE-PEG5000 coated TiO2 had not caused the significant decrease of spleen and thymus indexes. However, TiO2 caused appreciable toxicity in liver at the concentration of 1 mg mL−1 by blood markers analysis and histological assessment. By oxidative stress response, we found that TiO2 could induce the increase of SOD and the decrease of MDA after 24 h, but it recovered after 7 days. These findings will provide some useful information to TiO2 in nanotoxicology.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 81000668, 11304220 and 81471786), Natural Science Foundation of Tianjin (Grant no. 13JCQNJC13500), the Subject Development Foundation of Institute of Radiation Medicine, CAMS (Grant no. SF1440, SZ1336).References
- B. Trouiller, R. Reliene, A. Westbrook, P. Solaimani and R. H. Schiestl, Cancer Res., 2009, 69, 8784 CrossRef CAS PubMed.
- J. Wang, G. Zhou, C. Chen, H. Yu, T. Wang, Y. Ma, G. Jia, Y. Gao, B. Li, J. Sun, Y. Li, F. Jiao, Y. Zhao and Z. Chai, Toxicol. Lett., 2007, 168, 176 CrossRef CAS PubMed.
- D. Macwan, P. N. Dave and S. Chaturvedi, J. Mater. Sci., 2011, 46, 3669 CrossRef CAS.
- R. H. Crabtree, Science, 1998, 282, 2000 CrossRef CAS.
- Y. J. Hwang, C. Hahn, B. Liu and P. Yang, ACS Nano, 2012, 6, 5060 CrossRef CAS PubMed.
- H. Tang, K. Prasad, R. Sanjines, P. Schmid and F. Levy, J. Appl. Phys., 1994, 75, 2042 CrossRef CAS PubMed.
- L. Zeng, W. Ren, L. Xiang, J. Zheng, B. Chen and A. Wu, Nanoscale, 2013, 5, 2107 RSC.
- K. Sunada, T. Watanabe and K. Hashimoto, Environ. Sci. Technol., 2003, 37, 4785 CrossRef CAS.
- B. D. Johnston, T. M. Scown, J. Moger, S. A. Cumberland, M. Baalousha, K. Linge, R. van Aerle, K. Jarvis, J. R. Lead and C. R. Tyler, Environ. Sci. Technol., 2010, 44, 1144 CrossRef CAS PubMed.
- Z. Liu, W. Cai, L. He, N. Nakayama, K. Chen, X. Sun, X. Chen and H. Dai, Nat. Nanotechnol., 2007, 2, 47 CrossRef CAS PubMed.
- X. D. Zhang, J. Chen, Z. Luo, D. Wu, X. Shen, S. S. Song, Y. M. Sun, P. X. Liu, J. Zhao, S. Huo, S. Fan, F. Fan, X. J. Liang and J. Xie, Adv. Healthcare Mater., 2014, 3, 133 CrossRef CAS PubMed.
- X. D. Zhang, Z. Luo, J. Chen, X. Shen, S. Song, Y. Sun, S. Fan, F. Fan, D. T. Leong and J. Xie, Adv. Mater., 2014, 26, 4565 CrossRef CAS PubMed.
- B. D. Chithrani, A. A. Ghazani and W. C. Chan, Nano Lett., 2006, 6, 662 CrossRef CAS PubMed.
- A. Nel, T. Xia, L. Mädler and N. Li, Science, 2006, 311, 622 CrossRef CAS PubMed.
- W. -S. Cho, M. Cho, J. Jeong, M. Choi, H. -Y. Cho, B. S. Han, S. H. Kim, H. O. Kim, Y. T. Lim, B. H. Chung and J. Jeong, Toxicol. Appl. Pharmacol., 2009, 236, 16 CrossRef CAS PubMed.
- M. V. Park, A. M. Neigh, J. P. Vermeulen, L. J. de la Fonteyne, H. W. Verharen, J. J. Briedé, H. van Loveren and W. H. de Jong, Biomaterials, 2011, 32, 9810 CrossRef CAS PubMed.
- K. Suttiponparnit, J. Jiang, M. Sahu, S. Suvachittanont, T. Charinpanitkul and P. Biswas, Nanoscale Res. Lett., 2011, 6, 1 Search PubMed.
- L. Cheng, K. Yang, Y. Li, X. Zeng, M. Shao, S.-T. Lee and Z. Liu, Biomaterials, 2012, 33, 2215 CrossRef CAS PubMed.
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115 CrossRef CAS PubMed.
- C. Ispas, D. Andreescu, A. Patel, D. V. Goia, S. Andreescu and K. N. Wallace, Environ. Sci. Technol., 2009, 43, 6349 CrossRef CAS.
- E. J. Park, H. W. Shim, G. H. Lee, J. H. Kim and D. W. Kim, Arch. Toxicol., 2013, 87, 1219 CrossRef CAS PubMed.
- D. B. Warheit, T. R. Webb, C. M. Sayes, V. L. Colvin and K. L. Reed, Toxicol. Sci., 2006, 91, 227 CrossRef CAS PubMed.
- C. Jin, Y. Tang, F. G. Yang, X. L. Li, S. Xu, X. Y. Fan, Y. Y. Huang and Y. J. Yang, Biol. Trace Elem. Res., 2011, 141, 3 CrossRef CAS PubMed.
- E. Fabian, R. Landsiedel, L. Ma-Hock, K. Wiench, W. Wohlleben and B. van Ravenzwaay, Arch. Toxicol., 2008, 82, 151 CrossRef CAS PubMed.
- H. Liu, L. Ma, J. Zhao, J. Liu, J. Yan, J. Ruan and F. Hong, Biol. Trace Elem. Res., 2009, 129, 170 CrossRef CAS PubMed.
- J. Chen, X. Dong, J. Zhao and G. Tang, J. Appl. Toxicol., 2009, 29, 330 CrossRef CAS PubMed.
- S. Hussain, K. Hess, J. Gearhart, K. Geiss and J. Schlager, Toxicol. in Vitro, 2005, 19, 975 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang, S. Dubonos, I. Grigorieva and A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- X. Li, X. Wang, L. Zhang, S. Lee and H. Dai, Science, 2008, 319, 1229 CrossRef CAS PubMed.
- B. Y. Xia, B. Wang, H. B. Wu, Z. Liu, X. Wang and X. W. D. Lou, J. Mater. Chem., 2012, 22, 16499 RSC.
- B. Y. Xia, H. B. Wu, J. S. Chen, Z. Wang, X. Wang and X. W. D. Lou, Phys. Chem. Chem. Phys., 2012, 14, 473 RSC.
- B. Y. Xia, S. Ding, H. B. Wu, X. Wang and X. Wen, RSC Adv., 2012, 2, 792 RSC.
- Z. Liu, S. M. Tabakman, Z. Chen and H. Dai, Nat. Protoc., 2009, 4, 1372 CrossRef CAS PubMed.
- J. Chen, H. Wang, W. Long, X. Shen, D. Wu, S. S. Song, Y. M. Sun, P. X. Liu, S. Fan, F. Fan and X. D. Zhang, Int. J. Nanomed., 2013, 8, 2409 Search PubMed.
- X. D. Zhang, D. Wu, X. Shen, P. X. Liu, N. Yang, B. Zhao, H. Zhang, Y. M. Sun, L. A. Zhang and F. Y. Fan, Int. J. Nanomed., 2011, 6, 2071 CrossRef CAS PubMed.
- G. S. Hong, Y. P. Zou, A. L. Antaris, S. Diao, D. Wu, K. Cheng, X. D. Zhang, C. X. Chen, B. Liu, Y. H. He, J. Z. Wu, J. Yuan, B. Zhang, Z. M. Tao, C. Fukunaga and H. J. Dai, Nat. Commun., 2014, 5, 4206 Search PubMed.
- G. S. Hong, J. C. Lee, J. T. Robinson, U. Raaz, L. M. Xie, N. F. Huang, J. P. Cooke and H. J. Dai, Nat. Med., 2012, 18, 1841 CrossRef CAS PubMed.
- Z. Liu, S. M. Tabakman, Z. Chen and H. J. Dai, Nat. Protoc., 2009, 4, 1372 CrossRef CAS PubMed.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS PubMed.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888 CrossRef CAS PubMed.
- D. B. Chithrani, S. Jelveh, F. Jalali, M. van Prooijen, C. Allen, R. G. Bristow, R. P. Hill and D. A. Jaffray, J. Radiat. Res., 2010, 173, 719 CrossRef CAS PubMed.
- X. D. Zhang, J. Chen, Y. Min, G. B. Park, X. Shen, S. S. Song, Y. M. Sun, H. Wang, W. Long and J. Xie, Adv. Funct. Mater., 2014, 26, 1718 CrossRef PubMed.
- X. D. Zhang, D. Wu, X. Shen, J. Chen, Y. M. Sun, P. X. Liu and X. J. Liang, Biomaterials, 2012, 33, 6408 CrossRef CAS PubMed.
- X. D. Zhang, D. Wu, X. Shen, P.-X. Liu, F. Y. Fan and S. J. Fan, Biomaterials, 2012, 33, 4628 CrossRef CAS PubMed.
- S. Gui, B. Li, X. Zhao, L. Sheng, J. Hong, X. Yu, X. Sang, Q. Sun, Y. Ze, L. Wang and F. Hong, J. Agric. Food Chem., 2013, 61, 8959 CrossRef CAS PubMed.
- M. Lundqvist, J. Stigler, G. Elia, I. Lynch, T. Cedervall and K. A. Dawson, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14265 CrossRef CAS PubMed.
- C. Michiels, M. Raes, O. Toussaint and J. Remacle, Free Radical Biol. Med., 1994, 17, 235 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |