Templated magnesiothermic synthesis of silicon nanotube bundles and their electrochemical performances in lithium ion batteries†
Abstract
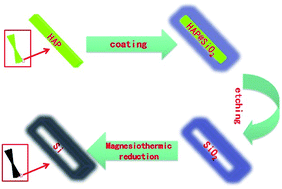
Silicon nanotube bundles (Si NBs) have been synthesized on a large scale via a templated magnesiothermic method. Hydroxyapatite (HAP) nanobelt bundles were fabricated and used as a sacrificial template to form silica nanotube bundles (SiO2 NBs) followed by transformation of SiO2 NBs into Si NBs through magnesiothermic reduction. The structure and morphology of the as-prepared Si NBs were examined in detail by XRD, Raman spectroscopy, SEM and TEM. The electrochemical application of Si NBs as an anode in lithium ion batteries (LIBs) has been investigated, which shows a reversible capacity with little fading, outweighing the performance using Si nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...