Benzocyclobutene resin with fluorene backbone: a novel thermosetting material with high thermostability and low dielectric constant
Abstract
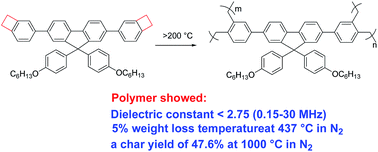
A fluorene-based monomer (FB) with thermally cross-linkable benzocyclobutene groups is reported here. This monomer showed good solubility in the common organic solvents and had a low melting point (128 °C). When being treated at high temperature (>200 °C), the monomer was converted to a cross-linked network structure (PFB). TGA data exhibited that PFB had high thermostability with a 5% weight loss temperature of 437 °C and 372 °C in N2 and air, respectively. Moreover, PFB showed a char yield of 47.6% at 1000 °C in N2. With regard to the electrical properties, PFB indicated an average of dielectric constants of about 2.7 ranging from 0.15 MHz to 30 MHz. All these results suggest that FB could be used as the varnish for insulating enameled wire in the electrical industry, and as encapsulation resins in the microelectronics industry.

 Please wait while we load your content...
Please wait while we load your content...