Controlling elastico-mechanoluminescence in diphase (Ba,Ca)TiO3:Pr3+ by co-doping different rare earth ions†
Jun-Cheng Zhang*aef,
Yun-Ze Longae,
Xusheng Wangb and
Chao-Nan Xu*cd
aCollege of Physics, Qingdao University, Qingdao 266071, P. R. China. E-mail: jc-zhang@qdu.edu.cn
bFunctional Materials Research Laboratory, Tongji University, Shanghai 200092, P. R. China
cNational Institute of Advanced Industrial Science and Technology, Kyushu, Shuku 807-1, Tosu, Saga 841-0052, Japan. E-mail: cn-xu@aist.go.jp
dInternational Institute for Carbon Neutral Energy Research (WPI-I2CNER), Kyushu University, Fukuoka, Japan
eKey Laboratory of Photonics Materials and Technology in Universities of Shandong, Qingdao University, Qingdao 266071, P. R. China
fShanghai Key Laboratory of Special Artificial Microstructure Materials and Technology, Tongji University, Shanghai 200092, P. R. China
First published on 18th August 2014
Abstract
Elastico-mechanoluminescence (EML) of diphase (Ba,Ca)TiO3:Pr3+,RE (RE includes all rare-earth-ions except Sc and Pm) is systematically investigated. The EML intensity is obviously controlled by the co-doped RE ions. Y, La, Nd, Gd, Yb, and Lu have the positive effect of improving EML, among which Gd enhances the EML intensity 61% at least. The depth and concentration of traps in (Ba,Ca)TiO3:Pr3+,RE are estimated according to the thermoluminescence curves. The consistent correlation between the dependence of EML intensity and trap concentration on the RE ions shows that the change of trap concentration induced by co-doping regulates the EML performance. Furthermore, the similar variations between the EML and afterglow (AG) intensities with the RE ions suggest the possibility that the same types of traps participate in the EML and AG processes. An EML mechanism is proposed on the basis of electrons as the main charge carriers.
1. Introduction
Mechanoluminescent (ML) materials, a specific type of solid phosphors, can convert local mechanical energy into light emission with the application of any mechanical stimulus.1,2 As a category of ML materials, elastico-mechanoluminescent (EML) materials present an accurate linearity of ML intensity against load in the elastic deformation range, in addition to the mechano-optical conversion.3,4 The potential of EML materials has been recognized as stress probes to monitor the stress distribution in artificial skin and bone, engineering structures, and living body in view of the advantages such as non-destruction, reproducibility, real-time, and reliability.5–12 Thus, research for EML materials has continuously gained popularity. More than twenty kinds of inorganic EML materials with different emission spectra from ultraviolet to infrared light have been successfully developed since ZnS:Mn2+ and SrAl2O4:Eu2+ were firstly reported in 1999.5,13 Nevertheless, the number of intense EML materials is quite limited. Among them, SrAl2O4:Eu2+ and ZnS:Mn/Mn,Te/Cu/Cu,Mn have relatively more intense EML.5,13–17 The brightness can be seen even in day light with the naked eye. Unfortunately, the aluminates and sulfides are chemically unstable, and, in particular, very sensitive to moisture, which greatly limits the scope of applications. Accordingly, the water-resistant EML materials, such as silicates SrCaMgSi2O7:Eu2+ and Ca2MgSi2O7:Eu2+, aluminosilicates Ca2Al2SiO7:Ce3+ and CaAl2Si2O8:Eu2+, have been synthesized, but their EML intensities are much weaker than that of SrAl2O4:Eu2+.18–21 Stable and intense EML materials are thus urgently needed.Recently, an intense EML has been reported in diphase (Ba,Ca)TiO3:Pr3+, which is considered one of the promising candidates for the EML applications. The most intense EML brightness achieved at the 60 mol% Ca content is above 15 mcd m−2, roughly 5000 times the light perception of dark-adapted eye (0.32 mcd m−2).22,23 More importantly, the host of this EML material has excellent chemical stability and superior water-resistant behavior.24 Therefore, to further enhance the EML intensity of diphase (Ba,Ca)TiO3:Pr3+ has very important value to realize the practical application in outdoor and day-light environments.
The interesting multifunctional phenomenon of EML materials provides us a clue to optimize EML performance. The developed EML materials including diphase (Ba,Ca)TiO3:Pr3+ are also long afterglow phosphors (LAPs). As indicated by the previous investigations, both of EML materials and LAPs belong to the defect-controlled type of luminescent materials.2–14,25 The EML and afterglow (AG) performances are closely related to the trap properties, especially the depth and concentration of trap levels. It is well known that rare earth ions co-doping is an effective concept in regulating the trap properties. It has been presented that the persistent luminescence of LAPs is strongly enhanced by co-doping with selected rare earth ions, e.g. blue LAPs CaAl2O4:Eu2+,Nd3+ and Sr2MgSi2O7:Eu2+,Dy3+, green LAPs SrAl2O4:Eu2+,Dy3+ and CaGa2S4:Eu2+,Ho3+, and red LAPs CaS:Eu2+,Tm3+ and Sr2SnO4:Sm3+,Dy3+.26–31 Up to now, countless reports have already been published on the influence of different rare earth ions co-doping on the persistent luminescence of LAPs in order to select the suitable co-doped rare earth ions.26,29,32 Accordingly, this method has also been used to enhance the EML intensity, but its validity has only been reported in a few cases of SrAl2O4:Eu2+,Dy3+, Ca2MgSi2O7:Eu2+,Dy3+, and SrAl2O4:Ce3+,Ho3+ co-doped by the special rare earth ions.19,33,34 Even so, less agreement exists on the role of the rare earth codopant. The introduced rare earth ions have been assumed to act as a new trap or to modify the trap properties, such as to deepen the trap depth or to increase trap concentration. The mechanism of EML obtained from the co-doped materials has also not been elucidated. Furthermore, the relation between AG and EML is still unclear. Although most authors agree on the existence of long-lived trap levels related to AG and EML, many details are still shrouded in mystery. In particular, it is wondered that whether the same types of traps are responsible for AG and EML. More importantly, the design and development of new EML materials would be greatly facilitated if the related mechanisms are revealed.
In the present work, different rare earth ions (including Y, La, Ce, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu) co-doped diphase (Ba,Ca)TiO3:Pr3+,RE with 60 mol% Ca were synthesized to search for suitable rare earth ions favoring the enhancement of EML intensity. The new introduced traps, trap depth and trap concentration in these materials were derived from the thermoluminescence (ThL) curves. The role of the rare earth codopant was studied. The nature of traps in (Ba,Ca)TiO3:Pr3+,RE as well as the co-doping effect on the photoluminescence (PL), AG, and EML were discussed in view of the results obtained. The intrinsic relation between AG and EML were assessed according to the similar variation trends among the ThL, AG and EML intensities with the co-doped rare earth ions. Finally, the EML mechanism of (Ba,Ca)TiO3:Pr3+,RE was proposed.
2. Experimental
Diphase (Ba,Ca)TiO3:Pr3+ (BCTP) with the formula (Ba0.4Ca0.6)0.998Pr0.002TiO3 and rare earth ions co-doped (Ba,Ca)TiO3:Pr3+,RE (BCTPRE) with the formula (Ba0.4Ca0.6)0.996Pr0.002RE0.002TiO3 (RE = Y, La, Ce, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu) were synthesized in air by the solid-state reaction method. Raw materials of BaCO3, CaCO3, TiO2, Pr6O11, and various trivalent rare earth oxides RE2O3 (≥99.9%) were mixed thoroughly and prefired at 900 °C for 4 h, then remixed, and subsequently sintered at 1400 °C for 4 h. To evaluate the EML property, the synthesized products were ground and screened through a 20 μm sieve, and then mixed in a transparent epoxy resin at a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (0.5 g
9 (0.5 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 g) to form the plastic disks 25 mm in diameter and 15 mm thick.
4.5 g) to form the plastic disks 25 mm in diameter and 15 mm thick.
The structural characterization was examined by X-ray diffraction (XRD, D8 Advance, Bruker AXS Gmbh). Photoluminescence (PL) was recorded with a fluorescence spectrometer (LS-55, Perkin-Elmer). The ThL curves were measured by combining a fluorescence spectrometer (FP-6600, Jasco Co.) with an in-house made temperature control unit. In order to actually compare the PL and ThL curves of different rare earth ions co-doped samples with those of (Ba,Ca)TiO3:Pr3+, the screened (Ba,Ca)TiO3:Pr3+ and (Ba,Ca)TiO3:Pr3+,RE powders with the same weight (0.5 g) were prepared. Compressive stress was applied on the composite disk with a universal testing machine (RTC-1310A, Orientec Corp.). The EML intensity was measured with a computer-driven photon-counting system that consists of a photomultiplier tube (R649, Hamamatsu Photonics K. K.) and a photon counter (C3866, Hamamatsu Photonics K. K.). Prior to the EML and ThL measurements, all the samples were irradiated at 254 nm for 1 min with a 6 W conventional ultraviolet (UV) lamp, and then the measurements were executed after the delay of 1 min. All measurements except ThL were performed at room temperature. All measurements were executed twice at least, and the experimental results have better repeatability.
3. Results and discussion
3.1 Crystallographic investigation of (Ba,Ca)TiO3:Pr3+,RE
Fig. 1 shows the powder XRD patterns of (Ba0.4Ca0.6)0.998Pr0.002TiO3 (BCTP) and (Ba0.4Ca0.6)0.996Pr0.002RE0.002TiO3 (BCTPRE). BCTP has a diphase coexistence of the tetragonal piezoelectric phase Ba0.77Ca0.23TiO3:Pr3+ and the orthorhombic phosphor phase Ba0.1Ca0.9TiO3:Pr3+. The stoichiometries of Ba0.77Ca0.23TiO3:Pr3+ and Ba0.1Ca0.9TiO3:Pr3+ have been calculated to be 44.8 mol% and 55.2 mol%, respectively. Our previous reports have indicated that EML in diphase (Ba,Ca)TiO3:Pr3+ comes from the interaction of sandwich architectures composed of piezoelectric phase and phosphor phase.22,23 The XRD patterns of (Ba,Ca)TiO3:Pr3+,RE do not contain any impure phase in comparison with that of (Ba,Ca)TiO3:Pr3+, indicating that low concentration of rare earth ions co-doping has no remarkable influence on the crystalline structure. | ||
| Fig. 1 XRD patterns of (Ba0.4Ca0.6)0.998Pr0.002TiO3 (BCTP) and (Ba0.4Ca0.6)0.996Pr0.002RE0.002TiO3 (BCTPRE, RE = Y, La, Ce, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu). | ||
3.2 Photoluminescence properties of (Ba,Ca)TiO3:Pr3+,RE
Fig. 2 shows the photoluminescence excitation (PLE, λem = 613 nm) and photoluminescence (PL, λex = 343 nm) spectra of BCTP and selected BCTPRE. All of the co-doped rare earth ions cause the decrease of PLE and PL intensities, but none of them were found to affect the locations of PLE and PL peaks, as well as the shape of spectra. The relative PL integral intensities of BCTPRE are plotted and compared in Fig. 3. Among them, Ce co-doping has the most serious quenching effect on the emission. | ||
| Fig. 2 (a) Photoluminescence excitation (PLE, λem = 613 nm) and (b) photoluminescence (PL, λex = 343 nm) spectra of (Ba,Ca)TiO3:Pr3+ (BCTP) and selected (Ba,Ca)TiO3:Pr3+,RE (BCTPRE). | ||
 | ||
| Fig. 3 Effect of rare earth ions co-doping on the photoluminescence (PL) integral intensity of (Ba,Ca)TiO3:Pr3+,RE. | ||
Fig. 2a shows that the excitation spectra are composed of four bands: two strong broad bands centered at ∼256 nm (A band) and ∼343 nm (B band), a weak broad band centered at ∼400 nm (C band), as well as the weak peaks (D band) at 456, 476, and 494 nm, respectively. These results are in good agreement with our previous reports on CaTiO3:Pr3+ and diphase (Ba,Ca)TiO3:Pr3+.35,36 A band is ascribed to the lowest field component of the 4f5d state of Pr3+. B band is assigned to the valence-to-conduction band transition [O(2p)–Ti(3d)]. C band is attributed to a low-lying Pr-to-metal (Pr3+–Ti4+) intervalence charge transfer state (CTS), by which photo-electrons are radiationlessly de-excited from the 3P0 state to the 1D2 state of Pr3+. The weak peaks (D band) at 456, 476, and 494 nm originate from 3H4 to 3PJ (J = 0, 1, 2) transitions of Pr3+, respectively. It is apparent that rare earth ions co-doping decreases the absorption of 4f 5d state of Pr3+ (A band) and host (B band), and no energy transfer from the co-doped rare earth ions to Pr3+ takes place.
Fig. 2b presents that the main emission peaks of BCTPRE all lie in 613 nm, which are ascribed to the 1D2–3H4 transition of Pr3+.35,36 Rare earth ions co-doping does not change the PL peak position and spectral shape. No rare earth ions or intrinsic defect related emission has been observed whether the excitation wavelength is 256, 343, 400, 456, 476 or 494 nm for BCTPRE, indicating that neither direct excitation of co-doped rare earth ions nor energy transfer from Pr3+ to co-doped rare earth ions occurs. It is well known that the energy absorbed by phosphors will be released mainly by three ways, i.e. light emission, energy transfer, and nonradiative transition. Therefore, the above results suggest that the co-doped rare earth ions could introduce trap centers to capture the excited charge carriers, delaying the recombination emission in Pr3+ and suppressing the PL intensity, or create nonradiative recombination centers to compete with Pr3+, quenching PL, rather than form new luminescence centers or act as sensitizers.
3.3 Elastico-mechanoluminescence properties of (Ba,Ca)TiO3:Pr3+,RE
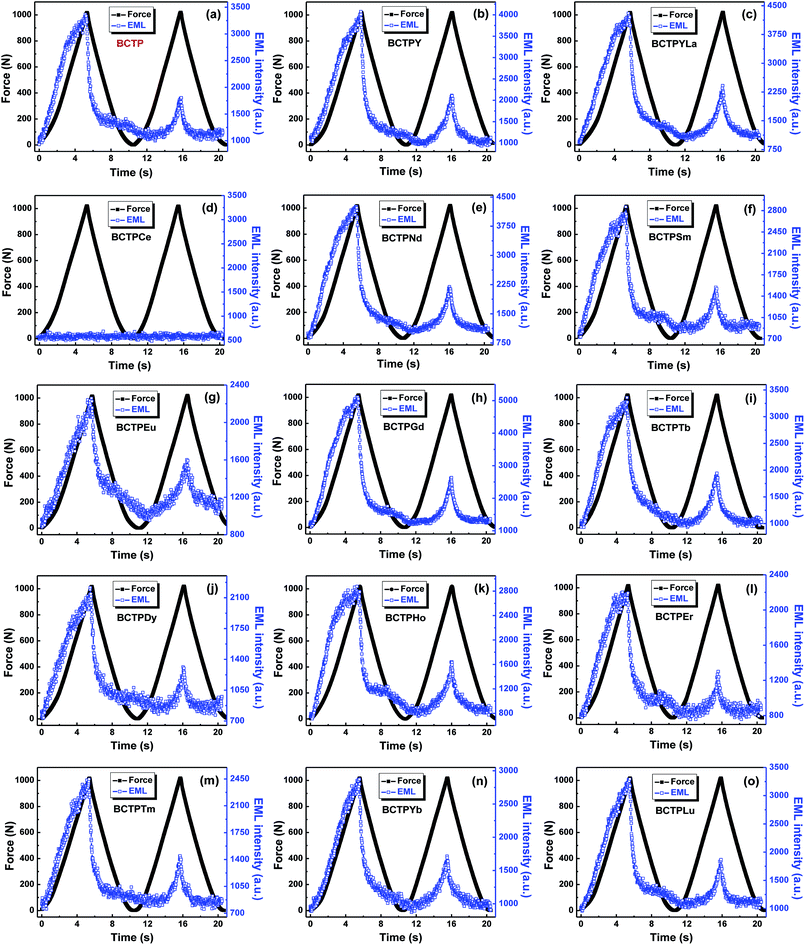
With a view to investigating the effect of rare earth ions co-doping on the EML performance, the EML behaviors of BCTP and BCTPRE are studied when a repetitive pressure up to 1000 N is applied at a rate of 3 mm min−1, as shown in Fig. 4. All of the EML curves except that of BCTPCe have a similar EML behavior. In each cycle, the EML intensity linearly changes with the increase of load, showing an EML peak at the peak load. When the load is released, the light is attenuated rapidly. As a repetitive pressure is applied, the EML peak intensity decreases obviously, which is ascribed to the de-trapping process of trapped carriers. It should be noted that the EML intensities of all these samples compressed repetitively will recover completely after the irradiation of UV light (254 nm) for 1 min, indicating the reproducibility of EML. The damage on their samples is very small after the repetitive mechanical experiments, which is useful for the practical application. These results are consistent with those of BCTP.22–24 However, rare earth ions have apparently different effects on EML. Only a few of rare earth ions (including Y, La, Nd, Gd, Yb, and Lu) enhance the EML intensity, while others have negative effect on EML. Similar with the case of PL, Ce co-doping also has a serious quenching effect on the EML of BCTP, resulting in the total disappearance of EML.The EML intensities of BCTP and BCTPRE for the 1st EML peak and the 2nd EML peak are compared and shown in Fig. 5a and b, respectively. It is evident that the EML intensity depends strongly on the type of co-doped rare earth ions. The co-doping effect on EML intensity has a similar change tendency with rare earth ions for the 1st and 2nd EML peaks. It is worthy of note that, different from the PL results, the EML intensity is improved prominently by several co-doped rare earth ions, including Y, La, Nd, Gd, Yb, and Lu. Among them, Gd and La have the most positive effect. For the 1st EML peak, about 60% enhancement of the EML intensity is obtained by Gd co-doping and about 40% enhancement for La co-doping, while for the 2nd EML peak, about 65% enhancement is obtained by Gd co-doping and about 70% enhancement for La co-doping. On the other hand, Sm, Eu, Dy, Er, and Tm quench EML in varying degrees, in addition to Ce inducing the disappearance of EML. The detailed parameters on the influence of rare earth ions co-doping in the EML intensity are listed in Table S1 (ESI).†
 | ||
| Fig. 5 Effect of rare earth ions co-doping on the EML intensity of (Ba,Ca)TiO3:Pr3+,RE: (a) for the 1st EML peak intensity and (b) for the 2nd EML peak intensity. | ||
Furthermore, as indicated in the section of experimental, the EML measurements were executed with the delay of 1 min after the UV irradiation. Thus, the EML intensity at the applied force of 0 N stands for the AG background (i.e. the long afterglow intensity) after stopping the UV irradiation for 1 minute. Fig. 6 shows the effect of rare earth ions co-doping on the AG background of (Ba,Ca)TiO3:Pr3+,RE. Interestingly, there are the similar variations for the EML intensity and AG background with the co-doped rare earth ions. The consistency indicates the EML and AG behaviors are closely related in (Ba,Ca)TiO3:Pr3+,RE, suggesting the possibility that the same types of traps participating in the processes of EML and AG.
3.4 Thermoluminescence analysis of (Ba,Ca)TiO3:Pr3+
Diphase (Ba,Ca)TiO3:Pr3+ belongs to defect-controlled type of EML materials, and the EML mechanism has been explained by a piezoelectrically induced trapped carrier de-trapping model, in which carriers (electrons or holes) trapped at the trap levels are released under the piezoelectric field induced by mechanical stimulus and then recombine with the luminescence centers, resulting in photon emission.22–24 Thus, the trap levels play an important role in the EML process. Thermoluminescence (ThL) technique is one of the most effective methods to determine the concentration as well as the depth (i.e. the activation energy) of trap levels in materials. The trap concentration is proportional to the area under the ThL curve. The trap depth E is proportional to corresponding ThL peak temperature. It can be estimated from the slope of the Hoogenstraaten plot and is expressed by the following equation:
E = −k![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(β/Tm2)/(1/Tm) ln(β/Tm2)/(1/Tm)
| (1) |
In the present study, the ThL curves of (Ba,Ca)TiO3:Pr3+ were measured from 83.15 K to 483.15 K at different heating rates of 10, 30, 60, and 90 K min−1. Before the measurement, UV lighting with a wavelength of 254 nm was irradiated on the sample for 1 min for charging the excitation electrons in the trap levels. The ThL curves are presented in Fig. 7. The shape of ThL curve is non-symmetric, suggesting multiple components. Accordingly, the ThL curves were analyzed by Gaussian deconvolution to better investigate the property of trap levels. The fitting result shows that there are two partly overlapping peaks, indicating that at least two kinds of traps exist in BCTP. The Hoogenstraaten plots for different fitting peaks allow us to derive E = 0.096 eV for Peak 1 and 0.356 eV for Peak 2 (Fig. 8). The ThL peaks close to (or above) room temperature are expected to be essential to EML and AG. The location of the Peak 1 is apparently far below room temperature (Fig. 7), thus the trap levels corresponding to Peak 1 will be emptied instantaneously at room temperature because of the shallow depth without contribution to the desired EML performance. The trap levels corresponding to Peak 2 with suitable depths could not be thermally activated at room temperature. They are responsible for the reproducible EML and AG phenomenon, but the traps related to EML are emptied faster than in the case of AG due to the application of mechanical stimulation.
 | ||
| Fig. 8 Hoogenstraaten plots for the different ThL peaks of (Ba,Ca)TiO3:Pr3+: (a) Peak 1; (b) Peak 2. | ||
3.5 Thermoluminescence analysis of (Ba,Ca)TiO3:Pr3+,RE
In order to investigate the co-doping effect on the properties of trap levels, the ThL measurements of (Ba,Ca)TiO3:Pr3+,RE (BCTPRE, RE = Y, La, Ce, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu) were also carried out under the same measurement conditions as that of (Ba,Ca)TiO3:Pr3+ (BCTP). Fig. 9 presents the ThL curves of BCTPRE at a heating rate of 90 K min−1. The ThL curves are fitted using Gaussian deconvolution and the dashed lines are the Gaussian components. The ThL curves of BCTPRE except those of BCTPCe and BCTPEr are composed of three Gaussian peaks (Peak 1, Peak 2, and Peak 3), indicating that at least three kinds of traps exist in BCTPRE. Comparing these ThL Gaussian peaks of BCTPRE with those of BCTP (Fig. 7a), in addition to two ThL peaks (Peak 1 and Peak 2) whose location and shape are almost the same as those of BCTP, there is a new low-temperature peak (Peak 3) located at about 160 K for BCTPRE. These results illuminate that rare earth ions co-doping indeed introduces a new kind of trap levels into the phosphors. However, the location of Peak 3 is far below room temperature, thus these trap levels will be emptied at or above room temperature, i.e. without contribution to EML. The same explanation applies to Peak 1. It should be pointed out that for BCTPCe, only the addition of Ce completely suppresses the ThL Peak 2, inducing the disappearance of EML (Fig. 4d). This phenomenon also confirms that Peak 2 in the ThL curve is responsible for the EML process. For BCTPEr, Er co-doping has no effect on introducing new ThL peak. There are still two ThL peaks (Peak 1 and Peak 2), which is similar with BCTP.Considering the dominant role of Peak 2 in the EML process of BCTPRE, the depth of trap levels corresponding to Peak 2 was estimated according to the above-mentioned Hoogenstraaten method. The effect of rare earth ions co-doping on the trap depth and relative ThL intergral intensity corresponding to Peak 2 of (Ba,Ca)TiO3:Pr3+,RE are plotted in Fig. 10 and 11, respectively. Table S2 (ESI)† presents these calculated trap depths and ThL intergral intensities of BCTPRE. Obviously, the trap depth seems to be less affected by rare earth ions (except Ce) co-doping. The trap depths of BCTPRE (except BCTPCe) are in the range of 0.30 to 0.38 eV, which are close to that of BCTP (0.356 eV). No correlation was found between the variation trends of trap depth and EML intensity with co-doped rare earth ion when comparing Fig. 10 and 6. In contrast, the ThL intensity is strongly influenced by co-doping. The ThL intensity is enhanced by the Y, La, Nd, Gd, Yb, and Lu co-dopings. As noted above, these rare earth ions co-dopings have the same positive effect on the improvement of EML intensity. More importantly, it is apparently observed that the variation of the ThL intensity with co-doped rare earth ion (Fig. 11) agrees well with that of the EML intensity (Fig. 6). Therefore, it is concluded that the change of trap concentration induced by rare earth ions co-doping has a more significant impact on regulating the EML performance of BCTPRe than that of trap depth. A similar direct correlation has also been found between the long afterglow luminescence and ThL intensity in CaAl2O4:Eu2+,RE3+.26
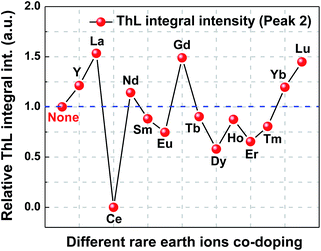 | ||
| Fig. 11 Effect of rare earth ions co-doping on the relative ThL intergral intensity (Peak 2) of (Ba,Ca)TiO3:Pr3+,RE. | ||
3.6 Elastico-mechanoluminescence mechanism of (Ba,Ca)TiO3:Pr3+,RE
It is well known that Pr3+ can be oxidized to Pr4+ when sintered in air, while Ti4+ would get the electron released by Pr3+ and be reduced to Ti3+, i.e. Pr3+ − e → Pr4+ and Ti4+ + e → Ti3+, or Pr3+ + Ti4+ → Pr4+ + Ti3+. Thus, several kinds of defects were formed during the synthesis process of (Ba,Ca)TiO3:Pr3+, including calcium and/or barium vacancies ([VCa/Ba]′′) to compensate [PrCa/Ba]o, Pr4+ ([PrCa/Ba]oo) which tends to form by oxidization of Pr3+ after thermal treatment in air, and negatively charged centers like Ti3+ ([TiTi]′) and/or interstitial oxygen [Oi]′′ correlated with the presence of Pr4+.38–40 However, except [PrCa/Ba]o as the electron trapping center which participates in the processes of AG and EML, the other defects which are not anticipated from the nominal chemical formulae act as nonradiative recombination centers.23,35In this study, (Ba,Ca)TiO3:Pr3+,RE were synthesized using various trivalent rare-earth oxides RE2O3 for the raw materials at high temperature (1400 °C) in air. A few of rare earth ions can be easily oxidized into tetravalent state or coexistence of different valence states, including Ce–Ce4+, Tb–Tb3+/Tb4+, and Dy–Dy3+/Dy4+, while other rare earth ions are mostly trivalent, i.e. Y3+, La3+, Nd3+, Sm3+, Eu3+, Gd3+, Ho3+, Er3+, Tm3+, Yb3+, and Lu3+.41 It is considered that RE3+ and RE4+ are incorporated into Ca2+ or Ba2+ sites according to the acceptable ion radius percentage difference (<30%) between the doped and substituted ions.42 Therefore, in the case of (Ba,Ca)TiO3:Pr3+,RE, besides those above-mentioned defects in (Ba,Ca)TiO3:Pr3+, more point defects arising from the rare earth ions impurity ([RECa/Ba]o and [RECa/Ba]oo) and the Ca or Ba vacancy ([VCa/Ba]′′) can be created in the host lattice due to charge compensation. Accordingly, the significant suppress of PL in (Ba,Ca)TiO3:Pr3+,RE (Fig. 2 and 3) should be attributed to the formation of more defects hampering the process of energy transfer to Pr3+. It is a typical example that the addition of Ce4+ which is known to be a luminescence killer causes a drastic quenching of PL in the several kinds of phosphors.43–45
On the other hand, these introduced defects also provide the possibility for regulating the EML properties of (Ba,Ca)TiO3:Pr3+,RE (Fig. 5 and Table S1†). The results of Fig. 11 and Table S2† have indicated that the EML intensity is strongly influenced by the ThL intensity of Peak 2. In (Ba,Ca)TiO3:Pr3+, the trap corresponding to the ThL Peak 2 has been ascribed to the electron trapping center [PrCa/Ba]o, which is responsible for the EML process.21 In (Ba,Ca)TiO3:Pr3+,RE, however, rare earth ions (except Ce) co-doping have less influence on the trap depth corresponding to ThL Peak 2. Therefore, rare earth ions co-doping acts upon regulating the EML intensity through changing the concentration of electron trapping center related to [PrCa/Ba]o, i.e. [RECa/Ba]o introduced by co-doping. It should be noted that the co-doped Ce mainly forms the [CeCa/Ba]oo defect in (Ba,Ca)TiO3:Pr3+,Ce prepared in air because of its low reduction potential. The total quenching of EML in Ce do-doped sample suggests the rare earth ions with low reduction potentials (i.e. easily be oxidized to tetravalent state), such as Ce and Dy, present the negative effect on EML (Fig. 5 and Table S1†). The ability of the rare earth ions to trap electron is probably based on the different reduction potentials. This conclusion can also be confirmed by the result that the most stable rare earth ions (e.g. La, Gd, Lu) with high reduction potentials have the most positive effect on EML (Fig. 5 and Table S1†). Nevertheless, the less effect of Tb co-doping on EML possibly arises from the instability of Tb4+ in (Ba,Ca)TiO3:Pr3+,Tb.
However, it is still uncertain why different RE3+ ions do not have the similar behavior on regulating EML. Because of their rather similar chemical properties, the effect of the RE3+ ions should be similar and thus the physical properties, mainly the ionization potentials and the 4f and 5d energy levels may play an important role. The effect of the differences in ionic size and bonding characteristics as well as some redox properties also cannot be excluded. Nevertheless, no correlation was found between the trap depths and the RE3+ level locations in this study. The effect of the RE3+ co-doping seems to be composed of several factors. The small differences in different factors will finally lead to the large differences observed in the efficiency of EML. Further studies are needed.
Fortunately, it is clear that the increase of EML from the Y, La, Nd, Gd, Yb, and Lu co-doped samples should be attributed to the new introduced electron trapping centers [RECa/Ba]o which have the similar trap depths with [PrCa/Ba]o participating in the EML process. On the other hand, according to the PL, AG and EML results, the [RECa/Ba]o defects formed by the other RE3+ ions co-doping possibly act as the nonradiative recombination centers which are adjacent to [PrCa/Ba]o to compete with [PrCa/Ba]o, resulting in the EML decrease. The detailed discussion is out of the scope of this work and will be presented elsewhere.
According to the results previously reported and obtained in this work,22–24,35,36 the EML processes of (Ba,Ca)TiO3:Pr3+ (before co-doping) and (Ba,Ca)TiO3:Pr3+,RE (after co-doping) are explained based on electrons as the main charge carriers as follows. Fig. 12 shows the schematic diagram of EML processes before and after co-doping.
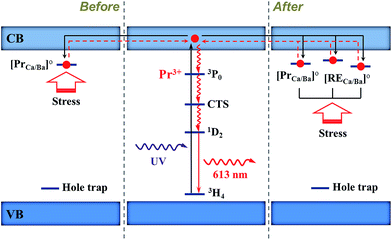 | ||
| Fig. 12 Proposed elastico-mechanoluminescence mechanism before and after co-doping, showing the possible trapping and de-trapping processes of electrons. CTS: charge transfer state. | ||
(1) Before co-doping. When (Ba,Ca)TiO3:Pr3+ is irradiated with UV light (254 nm), the electrons are firstly excited from the 3H4 ground level of Pr3+ to the conduction band of phosphor phase Ba0.1Ca0.9TiO3:Pr3+. Pr3+ stays now as Pr3+–h+ ionic complex. Then the electrons are trapped by the electron traps ([PrCa/Ba]o). When the stress is applied, the local piezoelectric field induced by piezoelectric phase Ba0.77Ca0.23TiO3:Pr3+ acts on the phosphor phase and leads to the de-trapping of trapped electrons to conduction band. Subsequently, these electrons radiationlessly de-excite from CTS via the 3P0 state to the 1D2 level of Pr3+–h+. Finally, the relaxation of the electrons back to the ground level of Pr3+ emits a red light of 613 nm.
(2) After co-doping. More electron trapping centers [RECa/Ba]o (RE = Y, La, Nd, Gd, Yb, Lu) are formed in the host. These traps have the similar depth with [PrCa/Ba]o. When the electrons of Pr3+ are excited to the conduction band, these electrons could be trapped by [PrCa/Ba]o and [RECa/Ba]o which is adjacent to [PrCa/Ba]o. Under the application of stress, in contrast to the case before co-doping, more trapped electrons are released from the traps of [PrCa/Ba]o and [RECa/Ba]o and then come back to luminescent center Pr3+, resulting in more intense EML. Alternatively, other positively charged defects including [RECa/Ba]o and [RECa/Ba]oo (RE = Ce, Sm, Eu, Dy, Er, Tm) might be created as nonradiative recombination centers after co-doping, hampering the EML process. This process is not depicted in Fig. 12.
It is important to note that Pr3+ has stronger natural tendency to be oxidized to Pr4+ than to be reduced to Pr2+, especially in (Ba,Ca)TiO3:Pr3+ sintered in air. In this work, therefore, electrons are the main charge carriers in the EML mechanism.
4. Conclusions
EML in diphase (Ba,Ca)TiO3:Pr3+ co-doped by various rare earth ions was systematically investigated. The EML intensities of (Ba,Ca)TiO3:Pr3+,RE are controlled by the co-doped RE ions. The Gd co-doping has the most positive effect on EML and the EML intensity is enhanced 61% at least. The similar variations were found between the EML and AG intensities with the RE ions, suggesting the possibility that the same types of traps responsible for EML and AG. The ThL curve of (Ba,Ca)TiO3:Pr3+,RE was used as a reference to study the effect of the rare earth ions co-doping on the properties of traps. A direct correlation between the EML intensity and high-temperature ThL peak intensity was found. The trap depths were affected by co-doping but not as much as the ThL intensity. The results indicate that the change of trap concentration induced by co-doping regulates the EML performance of (Ba,Ca)TiO3:Pr3+,RE. Finally, the EML mechanism of (Ba,Ca)TiO3:Pr3+,RE are explained based on electrons as the main charge carriers.Acknowledgements
This work was supported by the National Natural Science Foundation of China (11404181, 51072136 and 51373082), the Shandong Provincial Natural Science Foundation, China (ZR2013EMQ003), the Program of Science and Technology in Qingdao City (13-1-4-195-jch), the Opening Project of Shanghai Key Laboratory of Special Artificial Microstructure Materials and Technology (ammt2013A-2), the Natural Science Foundation of Shandong Province for Distinguished Young Scholars (JQ201103), the Taishan Scholars Program of Shandong Province (ts20120528), the Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province, and Grant-in-Aid for Scientific Research (A) (25249100) from JSPS.References
- J. Walton, Triboluminescence, Adv. Phys., 1977, 26(6), 887–948 CrossRef.
- B. P. Chandra, Mechanoluminescence, in Luminescence of Solids, ed.D. R. Vij, Plenum Press, New York, 1988 Search PubMed.
- B. P. Chandra and A. S. Rathore, Classification of mechanoluminescence, Cryst. Res. Technol., 1995, 30(7), 885–896 CrossRef CAS PubMed.
- C. N. Xu, Coatings, in Encyclopedia of Smart Materials, ed. M. Schwartz, Wiley, New York, 2002 Search PubMed.
- C. N. Xu, T. Watanabe, M. Akiyama and X. G. Zheng, Artificial skin to sense mechanical stress by visible light emission, Appl. Phys. Lett., 1999, 74(9), 1236–1238 CrossRef CAS PubMed.
- C. S. Li, C. N. Xu, L. Zhang, H. Yamada and Y. Imai, Dynamic visualization of stress distribution on metal by mechanoluminescence images, J. Visualization, 2008, 11(4), 329–335 CrossRef.
- N. Terasaki and C. N. Xu, Historical-log recording system for crack opening and growth based on mechanoluminescent flexible sensor, IEEE Sens. J., 2013, 13(1), 3999–4004 CrossRef.
- T. Z. Zhan, C. N. Xu, O. Fukuda, H. Yamada and C. S. Li, Direct visualization of ultrasonic power distribution using mechanoluminescent film, Ultrason. Sonochem., 2011, 18(1), 436–439 CrossRef CAS PubMed.
- N. Terasaki, H. Yamada and C. N. Xu, Ultrasonic wave induced mechanoluminescence and its application for photocatalysis as ubiquitous light source, Catal. Today, 2013, 201, 203–208 CrossRef CAS PubMed.
- J. C. Zhang, C. N. Xu, S. Kamimura, Y. Terasawa, H. Yamada and X. Wang, An intense elastico-mechanoluminescence material CaZnOS:Mn2+ for sensing and imaging multiple mechanical stresses, Opt. Express, 2013, 21(11), 12976–12986 CrossRef CAS PubMed.
- J. C. Zhang, C. N. Xu and Y. Z. Long, Elastico-mechanoluminescence in CaZr(PO4)2:Eu2+ with multiple trap levels, Opt. Express, 2013, 21(11), 13699–13709 CrossRef CAS PubMed.
- V. K. Chandra and B. P. Chandra, Dynamics of the mechanoluminescence induced by elastic deformation of persistent luminescent crystals, J. Lumin., 2012, 132(3), 858–869 CrossRef CAS PubMed.
- C. N. Xu, T. Watanabe, M. Akiyama and X. G. Zheng, Direct view of stress distribution in solid by mechanoluminescence, Appl. Phys. Lett., 1999, 74(17), 2414–2416 CrossRef CAS PubMed.
- Y. Liu and C. N. Xu, Influence of calcining temperature on photoluminescence and triboluminescence of europium-doped strontium aluminate particles prepared by sol-gel process, J. Phys. Chem. B, 2003, 107(17), 3991–3995 CrossRef CAS.
- C. N. Xu, H. Yamada, X. Wang and X. G. Zheng, Strong elasticoluminescence from monoclinic-structure SrAl2O4, Appl. Phys. Lett., 2004, 84(16), 3040–3042 CrossRef CAS PubMed.
- D. R. Reddy and B. K. Reddy, Laser-like mechanoluminescence in ZnMnTe-diluted magnetic semiconductor, Appl. Phys. Lett., 2002, 81(3), 460–462 CrossRef CAS PubMed.
- S. M. Jeong, S. Song, S. K. Lee and N. Y. Ha, Color manipulation of mechanoluminescence from stress-activated composite films, Adv. Mater., 2013, 25(43), 6194–6200 CrossRef CAS PubMed.
- H. Zhang, H. Yamada, N. Terasaki and C. N. Xu, Stress-induced mechanoluminescence in SrCaMgSi2O7:Eu, Electrochem. Solid-State Lett., 2007, 10(10), J129–J131 CrossRef CAS PubMed.
- H. Zhang, H. Yamada, N. Terasaki and C. N. Xu, Green mechanoluminescence of Ca2MgSi2O7:Eu and Ca2MgSi2O7:Eu,Dy, J. Electrochem. Soc., 2008, 155(2), J55–J57 CrossRef CAS PubMed.
- M. Akiyama, C. N. Xu, H. Matsui, K. Nonaka and T. Watanabe, Recovery phenomenon of mechanoluminescence from Ca2Al2SiO7:Ce by irradiation with ultraviolet light, Appl. Phys. Lett., 1999, 75(17), 2548–2550 CrossRef CAS PubMed.
- L. Zhang, H. Yamada, Y. Imai and C. N. Xu, Observation of elasticoluminescence from CaAl2Si2O8:Eu2+ and its water resistance behavior, J. Electrochem. Soc., 2008, 155(3), J63–J65 CrossRef CAS PubMed.
- X. Wang, C. N. Xu, H. Yamada, K. Nishikubo and X. G. Zheng, Electro-mechano-optical conversions in Pr3+-doped BaTiO3–CaTiO3 ceramics, Adv. Mater., 2005, 17(10), 1254–1258 CrossRef CAS PubMed.
- J. C. Zhang, X. Wang, X. Yao, C. N. Xu and H. Yamada, Strong elastico-mechanoluminescence in diphase (Ba,Ca)TiO3:Pr3+ with self-assembled sandwich architectures, J. Electrochem. Soc., 2010, 157(12), G269–G273 CrossRef CAS PubMed.
- J. C. Zhang, M. Tang, X. Wang, Y. Li and X. Yao, Elastico-mechanoluminescence properties of Pr3+-doped BaTiO3-CaTiO3 diphase ceramics with water resistance behavior, Ceram. Int., 2012, 38(S1), S581–S584 CrossRef CAS PubMed.
- J. Botterman, K. V. D. Eeckhout, I. D. Baere, D. Poelman and P. F. Smet, Mechanoluminescence in BaSi2O2N2:Eu, Acta Mater., 2012, 60(15), 5494–5500 CrossRef CAS PubMed.
- T. Aitasalo, J. Hälsö, H. Jungner, M. Lastusaari and J. Niittykoski, Thermoluminescence study of persistent luminescence materials: Eu2+- and R3+-doped calcium aluminates, CaAl2O4:Eu2+,R3+, J. Phys. Chem. B, 2006, 110(10), 4589–4598 CrossRef CAS PubMed.
- B. Liu, C. Shi, M. Yin, L. Dong and Z. Xiao, The trap states in the Sr2MgSi2O7 and (Sr,Ca)MgSi2O7 long afterglow phosphor activated by Eu2+ and Dy3+, J. Alloys Compd., 2005, 387(1), 65–69 CrossRef CAS PubMed.
- K. Korthout, K. V. D. Eeckhout, J. Botterman, S. Nikitenko, D. Poelman and P. F. Smet, Luminescence and X-ray absorption measurements of persistent SrAl2O4:Eu,Dy powders: evidence for valence state changes, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84(8), 085140 CrossRef.
- C. Guo, Q. Tang, D. Huang, C. Zhang and Q. Su, Influence of co-doping different rare earth ions on CaGa2S4:Eu2+,RE3+ (RE = Ln) phosphors, J. Phys. Chem. Solids, 2007, 68(2), 217–223 CrossRef CAS PubMed.
- D. Jia, W. Jia, D. R. Evans, W. M. Dennis and H. Liu, Zhu J.; Yen, W. M. Trapping processes in CaS: Eu2+,Tm3+, J. Appl. Phys., 2000, 88(6), 3402–3407 CrossRef CAS PubMed.
- X. Yu, X. Xu and J. Qiu, Enhanced long persistence of Sr2SnO4:Sm3+ red phosphor by co-doping with Dy3+, Mater. Res. Bull., 2011, 46(4), 627–629 CrossRef CAS PubMed.
- K. V. D. Eeckhout, P. F. Smet and D. Poelman, Persistent luminescence in rare-earth codoped image, J. Lumin., 2009, 129(10), 1140–1143 CrossRef PubMed.
- G. J. Yun, M. R. Rahimi, A. H. Gandomi, G. C. Lim and J. S. Choi, Stress sensing performance using mechanoluminescence of SrAl2O4:Eu (SAOE) and SrAl2O4:Eu, Dy (SAOED) under mechanical loadings, Smart Mater. Struct., 2013, 22(5), 055006 CrossRef.
- H. Zhang, H. Yamada, N. Terasaki and C. N. Xu, Ultraviolet mechanoluminescence from SrAl2O4:Ce and SrAl2O4:Ce,Ho, Appl. Phys. Lett., 2007, 91(8), 081905 CrossRef PubMed.
- J. C. Zhang, X. Wang and X. Yao, Enhancement of luminescence and afterglow in CaTiO3:Pr3+ by B site Zr substitution for Ti, J. Alloys Compd., 2010, 498(2), 152–156 CrossRef CAS PubMed.
- J. C. Zhang, W. Yang, X. Wang and X. Yao, Dielectric and luminescence properties of the A- and B-site doped CaTiO3:Pr3+ ceramics, Ferroelectrics, 2010, 401(1), 226–232 CrossRef CAS.
- W. Hoogenstraaten, Electron traps in zinc-sulfide phosphors, Philips Res. Rep., 1958, 13(6), 515–693 CAS.
- P. T. Diallo, P. Boutinaud, R. Mahiou and J. C. Cousseins, Red luminescence in Pr3+-doped calcium titanates, Phys. Status Solidi A, 1997, 160(1), 255–263 CrossRef CAS.
- P. Boutinaud, E. Pinel and R. Mahiou, Luminescence and afterglow in CaTiO3:Pr3+ films deposited by spray pyrolysis, Opt. Mater., 2008, 30(7), 1033–1038 CrossRef CAS PubMed.
- A. Zhu, J. Wang, D. Zhao and Y. Du, Native defects and Pr impurities in orthorhombic CaTiO3 by first-principles calculations, Phys. B, 2011, 406(13), 2697–2702 CrossRef CAS PubMed.
- F. M. A. Sroor and F. T. Edelmann, Lanthanides: Tetravalent Inorganic, in The Rare Earth Elements: Fundamentals and Applications, ed.D. A. Atwood, Wiley, New York, 2012 Search PubMed.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides, Acta Crystallogr., 1976, A32, 751–767 CrossRef CAS.
- A. Nag and T. R. N. Kutty, Photoluminescence of Sr2−xLnxCeO4+x/2 (Ln = Eu, Sm or Yb) prepared by a wet chemical method, J. Mater. Chem., 2003, 13(2), 370–376 RSC.
- A. Potdevina, G. Chadeyrona, V. Brioisc and R. Mahioub, Structural, morphological and scintillation properties of Ce3+-doped Y3Al5O12 powders and films elaborated by the sol–gel process, Mater. Chem. Phys., 2011, 130(1–2), 500–506 CrossRef PubMed.
- M. Kitsuda and S. Fujihara, Quantitative luminescence switching in CePO4:Tb by redox reactions, J. Phys. Chem. C, 2011, 115(17), 8808–8815 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05894a |
| This journal is © The Royal Society of Chemistry 2014 |