Controlled synthesis of ZnGa2O4 nanorod arrays from hexagonal ZnO microdishes and their photocatalytic activity on the degradation of RhB†
Zesheng Li*a,
Bolin Lia,
Zhenghui Liua,
Dehao Lia,
Chunyu Geb and
Yueping Fang*b
aDevelopment Center of Technology for Petrochemical Pollution Control and Cleaner Production of Guangdong Universities, College of Chemical Engineering, Guangdong University of Petrochemical Technology, Maoming, Guangdong 525000, China
bInstitute of Biomaterial, College of Science, South China Agricultural University, Guangzhou, 510642, China. E-mail: lzs212@163.com; ypfang@scau.edu.cn
First published on 22nd September 2014
Abstract
Novel ZnGa2O4 nanorod arrays with desirable photocatalytic activity for organic dye degradation have been synthesized by an associated solution–liquid–solid growth and topological morphology-conversion synthetic strategy.
The manipulation of physical dimensions and chemical compositions of semiconductors has attracted intensive attention due to their important roles in determining the physicochemical properties of the materials.1 The emergence and application of nanotechnology has provided great exciting advances in controlling the structure and morphology of semiconductors.2 Among various nanostructures, one-dimensional (1-D) nanostructures, such as wires, tubes, rods and belts have attracted intense research interest because they exhibit outstanding properties in the optical, electronic, and catalytic fields.3 Compared with conventional bulk materials and 0-D structures (e.g., nanoparticles), 1-D nanostructures can improve the transport of charge carriers and thus reduce the recombination losses at grain boundaries.4 Particularly, the aligned 1-D nanostructures formed by the directed self-assembly of nanorods, nanowires and nanotubes are a class of novel 1-D oriented architectures that gives rise to extraordinary collective properties.5 Such self-supported 1-D arrays are demonstrated to have high performance in catalysis systems for the photocatalytic degradation of organic dyes6 and for air purification7 due to their large surface area, integrated charge-transfer paths, as well as morphological stability.8
As an important functional semiconductor with a direct wide band gap (3.37 eV) and a large exciton binding energy (60 meV), ZnO has attracted great interest owing to its potential applications in photocatalysis, solar cells, sensors, nanogenerators, room-temperature ultraviolet (UV) lasers, optical waveguides, and so on.9 Recently, ZnGa2O4, a bimetallic semiconductor with spinel structure, has been investigated for its technical application in the photocatalytic field.10 Because its hybridized orbits of Ga4s4p and Zn4s4p and wider band gap (4.4 eV), ZnGa2O4 can promote the mobility of photogenerated electrons and improve the absorption efficiency in UV light radiation, which is the most available light source for waste water purification by photocatalytic degradation.11 Generally, 1-D ZnGa2O4 could be synthesized by chemical vapor deposition (CVD),12 which requires a thin-film ZnO template and multiple steps of high-temperature processes (>1000 °C). It is believed that complicated methods of material synthesis are not advantageous for further applications.13 For this purpose, a facile synthesis method for 1-D ZnGa2O4 nanostructures is highly desirable for their scalable application in photocatalytic fields.
It is well known that the solution–liquid–solid (SLS) growth mechanism has been widely applied to prepare various continuous 1-D crystalline nanostructures via hydrothermal synthesis routes, in which low-melting point metals (e.g., In, Ga and Sn, etc.) are used to induce the growth of 1-D nanostructures.14 Previously, we have developed the SLS method under hydrothermal conditions for the growth of Sn-filled In(OH)3 nanotubes15 and the twinned nanotowers and nanodendrites of HgSe.16 Recently, we further developed a Ga-mediated SLS growth strategy for the synthesis of novel Ga-doped, self-supported, independent, aligned ZnO nanorods by one-pot hydrothermal synthesis.17 Furthermore, the topological morphology conversion (TMC) process has proven to be a promising technique for the synthesis of newly nanostructured materials by means of chemical and structural transformation from established precursors with similar morphologies.18 Most recently, we reported the synthesis of novel SnO2-doped SiC hollow nanochains by TMC process from a precursor of SnO2@C core–shell nanochains.19
In this study, we demonstrate the controlled synthesis of ZnGa2O4 nanorod arrays from hexagonal ZnO microdishes by using an efficient strategy utilizing SLS growth and TMC process. Firstly, the Ga-doped ZnO nanorod arrays were synthesized by the regular Ga-mediated SLS hydrothermal synthesis,17 in which the hexagonal ZnO microdishes were transformed into aligned ZnO nanorods. Secondly, ZnGa2O4 nanorod arrays were further synthesized by a heat-treating TMC process from the Ga-doped ZnO nanorod arrays (see ESI† for detailed synthetic steps). The newly designed 1-D semiconductor nanostructures of ZnGa2O4 nanorod arrays showed a very high activity for the photocatalytic degradation of Rhodamine B (RhB) under UV light radiation.
The phase structures of the as-synthesized samples have been characterized by XRD. Fig. 1 shows the XRD patterns of the hexagonal ZnO microdishes (black pattern), Ga-doped ZnO nanorod arrays (red pattern) and the ZnGa2O4 nanorod arrays (blue pattern). From the black and red patterns, it can be readily concluded that the two samples have the hexagonal phase structures of ZnO (JCPDS card no. 36-1451 (ref. 9)). The sharp and clear diffraction peaks clearly indicate the good crystalline nature of the two products. It suggests that the ideal ZnO structures have been obtained by the hydrolyzation of ZnSt2 under hydrothermal treatment. Expressly, from the red pattern, no obvious Ga diffraction peak can be observed for the Ga-doped ZnO sample, which is mainly due to the amorphous existence of Ga dopant as indicated by one of our previous works.17 As illustrated by the blue pattern, the XRD pattern of the ZnGa2O4 sample can be indexed as cubic spinel ZnGa2O4 with a cell parameter of a = 8.335 Å, agreeing well with the JCPDS Card no. 38-1240.10 Several distinct peaks at 30.31, 35.71, 43.41, 57.41 and 63.01 match well with the (220), (311), (400), (511) and (440) crystal planes of ZnGa2O4, respectively. There is negligible ZnO component that remained in the ZnGa2O4 sample. Therefore, the results indicate that the ZnGa2O4 phase has been successfully synthesized from the Ga-doped ZnO phase via the heat-treating conversion process.
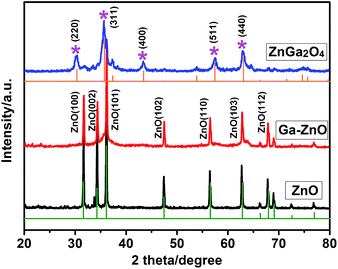 | ||
| Fig. 1 XRD pattern of hexagonal ZnO microdishes (black), Ga-ZnO nanorod arrays (red) and ZnGa2O4 nanorod arrays (blue). | ||
Schematic illustrations of crystal structures for ZnGa2O4 are illustrated in Fig. 2. The ball-and-stick model (Fig. 2A) shows the typical cubic unit cell of spinel ZnGa2O4, corresponding to the XRD data. Fig. 2B displays the crystallographic shear of ZnGa2O4, where zinc/oxygen tetrahedrons are shown in yellow. For one unit cell of ZnGa2O4, 32O2− constitute the close-packed subunit cell with a face-centred-cubic structure, 8Zn2+ occupy 8 tetrahedral vacancies (accounting for 1/8 of the total number of tetrahedral vacancies) to form 4 A-sites, and 16Ga3+ occupy 16 octahedral vacancies (accounting for 1/2 of the total number of octahedral vacancies) to form 4 B-sites, where one complete unit cell of spinel ZnGa2O4 is made up of the 4 A-sites and 4 B-sites by alternate permutation.
 | ||
| Fig. 2 Schematic illustrations of the crystal structures for ZnGa2O4: (A) a ball-and-stick model and (B) the crystallographic shear, zinc/oxygen tetrahedrons are showed in yellow. | ||
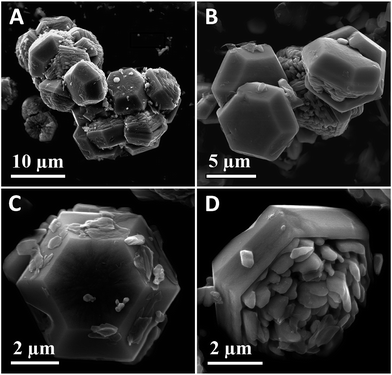
To investigate the detailed nanostructures and morphological evolution of the as-prepared samples, FE-SEM and TEM characterization were further implemented. Fig. 3 shows the representative SEM images of the hexagonal ZnO microdishes. The lower-magnification images (Fig. 3A and B) indicate that the sample has an interesting configuration comprising homologous 3-D hexagonal microdishes. The higher-magnification images (Fig. 3C and D) reveal that the flowerpot is made up of a hexagonal pot on one side and short nanorods on the other (just like one flowerpot).
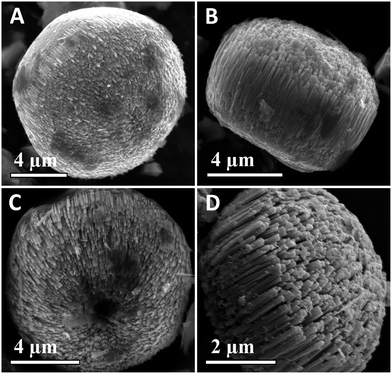
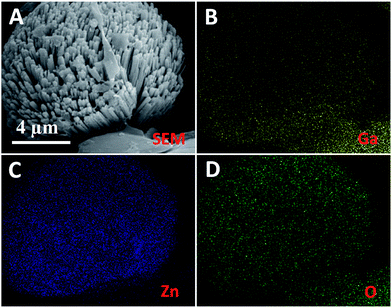
Fig. 4A and B show the representative SEM images of Ga-doped ZnO nanorod arrays. Evidently, the sample has highly self-supported, aligned 1-D nanostructures, where the ZnO nanorods are vertically well-assembled into micro-architectures without extraneous supporting substrates. These results demonstrate that ZnO 1-D nanostructures have been achieved by the Ga-mediated SLS hydrothermal process. Fig. 4C and D show the representative SEM images of ZnGa2O4 nanorod arrays, which reveal the loosely aligned 1-D nanostructures relative to the Ga-doped ZnO sample. Fig. 5 shows the SEM-EDS elemental mapping of Ga, Zn and O from the individual ZnGa2O4 nanorod array. It is found that the three elements, Ga, Zn and O (Fig. 5B–D), are homogeneously distributed, corresponding to the SEM image (Fig. 5A).
Fig. 6 shows the TEM images of Ga-doped ZnO nanorods (Fig. 6A and B) and ZnGa2O4 nanorods (Fig. 6C and D). It can be observed that the two samples both have dense, aligned nanorod architectures with single nanorods of 50–80 nm diameter. A spot of nanopores exists in the ZnGa2O4 nanorods (see Fig. 6D), which might originate from crystal defects during the structure conversion process. The insets in Fig. 6B and D further indicate the (101) planes of ZnO (d = 0.248 nm) and the (220) planes of ZnGa2O4 (d = 0.295 nm). The above-mentioned results largely demonstrate the feasibility of topological morphology conversion of Ga-doped ZnO nanorods to ZnGa2O4 nanorods by the TMC heat-treating process. In addition, the BET specific surface areas of ZnGa2O4 nanorods, Ga-doped ZnO nanorods and hexagonal ZnO microdishes were determined as 65.7, 61.2 and 38.4 m2 g−1, respectively.
The possible formation process of the ZnGa2O4 nanorod arrays, involving the evolution of Ga-doped ZnO nanorods and ZnGa2O4 nanorods, are schematically shown in Fig. 7. In the absence of Ga during the hydrothermal process, hexagonal ZnO microdishes were obtained (see Fig. 3 for details), indicating the Ga species plays an important role in the formation of the ZnO 1-D framework.17 Fig. 7 (i) illustrates a formation process of the Ga-doped ZnO nanorods with the SLS growth route. The SLS process may consist of four steps:14 formation of the liquid Ga droplets in hydrothermal solution (S), production of ZnO by ZnSt2 hydrolysis and its diffusion to liquid Ga droplet to form a liquid–solid (LS) interface, 1-D growth of the Ga-doped ZnO nanorods at the LS interface, and continuous 1-D growth of ZnO, consuming the liquid Ga droplet and thus completing the SLS assembly of the Ga-doped ZnO nanorods. Fig. 7 (ii) illustrates the latent evolution of the ZnGa2O4 nanorods from Ga-doped ZnO nanorods in the TMC process. In this process, the initial nanorod morphology of the Ga-doped ZnO precursor would be inherited by the ZnGa2O4 product by means of the potential chemical and structural transformation (ZnO reacting with Ga to form ZnGa2O4) under the heat-treating process. Further study on the accurate reaction mechanism is in progress.
The photocatalytic activities and stability of the as-prepared photocatalysts (the ZnGa2O4 nanorods, Ga-doped ZnO nanorods and hexagonal ZnO microdishes) were evaluated by degradation of RhB in aqueous solution under UV light radiation (see Fig. 8). Obviously, the ZnGa2O4 nanorods exhibit a much higher photocatalytic activity than the Ga-doped ZnO nanorods and hexagonal ZnO microdishes (Fig. 8A). RhB was largely degraded (97.2%) in 90 min under UV light radiation, while the degradation rates of Ga-doped ZnO nanorods and hexagonal ZnO microdishes were only 75.9% and 57.1%, respectively. The first-order reaction rate constant can be calculated by the plots of the ln(C/C0) vs. radiation time (t). The obtained rate law may be ln(C/C0) = −kt, where C is the concentration of dye, C0 the initial concentration of dye, k the reaction rate constant, and t the irradiation time. The degradation rate constant k of ZnGa2O4 nanorods was estimated to be 0.0397 min−1, which was 2.51 times and 4.22 times higher than those of Ga-doped ZnO nanorods (0.0158 min−1) and hexagonal ZnO microdishes (0.0094 min−1), respectively (Fig. 8B). Fig. 8C shows the change of absorption spectra of the RhB solution when exposed to UV light at different times in the presence of the ZnGa2O4 photocatalyst sample, which clearly shows that the absorption peak of RhB drops gradually with an increase of exposure time to 90 min (confirming the degradation of RhB as shown in Fig. 8A). Furthermore, stability testing on the ZnGa2O4 sample illustrates that the degradation rate shows slight decrease after four runs of irradiation within 360 min (Fig. 8D). The above mentioned results demonstrate that the obtained ZnGa2O4 nanorods can be used as a promising photocatalyst with excellent activity and desirable stability for the degradation of RhB in aqueous solution under UV light radiation.
The physical dimensions and chemical compositions of semiconductors have significant effects on their physicochemical properties.1 The smaller dimension of semiconductors often leads to higher photocatalytic activity because of their higher specific surface area along with optimized utilization efficiency.2 Obviously, the specific surface area of Ga-doped ZnO nanorods (61.2 m2 g−1) is much higher than that of hexagonal ZnO microdishes (38.4 m2 g−1). Hence, the improved activity of Ga-doped ZnO nanorods relative to hexagonal ZnO microdishes can be attributed to the high surface area of nanorod structures. However, the specific surface areas of ZnGa2O4 nanorods (65.7 m2 g−1) and Ga-doped ZnO nanorods (61.2 m2 g−1) are more approximate, so the high activity of the ZnGa2O4 nanorods sample is largely due to its favorable material structures.
It is generally accepted that the superior photocatalytic activity of ZnGa2O4 originates from its unique electronic and band structures. For one thing, the bottom of the conduction band (LUMO) is formed by Ga4s4p–Zn4s4p hybridized atomic orbits, which promote the mobility of photogenerated electrons and are beneficial for charge separation.20 For another, the wider band gap (4.4 eV) endows the photogenerated charge with stronger reductive capability, which results in stronger redox ability of the photogenerated electron–hole pairs, thus facilitating the photocatalytic activity.21 In addition, the possible existence of trace heterojunctions of ZnO–ZnGa2O4 may contribute to photocatalytic activity by the improved charge transfer. Accurate results are expected by further advanced characterization techniques.
To analyze the catalytic mechanism, the band structures of ZnO and ZnGa2O4 and possible pathway of RhB photocatalytic degradation are schematically illustrated in Fig. 9. In the case of ZnGa2O4, the EVB is 3.15 V, which is much higher than the 2.38 V of E(HO˙/OH−); the ECB is −1.25 V, which is much lower than the −0.33 V of E(O2/O2−˙).11 In the case of ZnO, only the ECB is lower than that of E(O2/O2−˙). As a result, HO˙ can be more readily generated for ZnGa2O4 under UV light in RhB aqueous solution, leading to the ZnGa2O4 photocatalysts' much higher photocatalytic properties than that of ZnO. The possible reaction process is as follows: once the ZnGa2O4 catalyst generates the electron–hole pairs by UV light radiation, the photogenerated electrons (e−) on the conduction band (CB) bond with adsorbed oxygen and water to form strong oxidizing species of O2−˙ and HO˙, while the holes (h+) on the valence band (VB) also bond with adsorbed oxygen and water to form HO˙. Under the action of substantial, strong oxidizing species, the structure of RhB is destroyed and finally decomposed into CO2 and H2O. All these processes might be described as follows:22
| e− + O2 → ˙O2− | (1) |
| ˙O2− + e− + 2H+ → H2O2 | (2) |
| H2O2 + e− → ˙OH + OH− | (3) |
| h+ + H2O → ˙OH + H+ | (4) |
| ˙OH + RhB → CO2 + H2O | (5) |
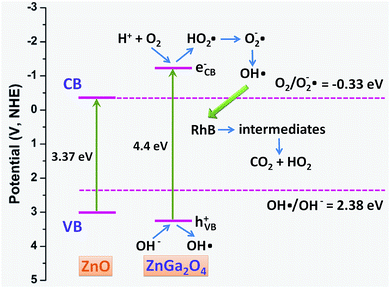 | ||
| Fig. 9 Schematic illustration for the band structures of ZnO and ZnGa2O4 and the proposed pathway of RhB photocatalytic degradation. | ||
It is well known that plenty of materials have been available for photocatalytic degradation of RhB, including TiO2,23 ZnO,24 AgBr,25 Ag3PO4,26 BiOBr,27 BiPO428 as well as ZnGa2O4. TiO2 is the most extensively studied and stable catalyst for photocatalytic degradation in the scientific perspective. However, it is difficult to determine which of them is the most promising photocatalyst in real-time application, due to their different catalytic mechanisms and the cost of materials. In the present study, we report the controlled synthesis of self-supported ZnGa2O4 nanorod arrays by using an efficient SLS growth and TMC process associated strategy. Although it is difficult to discuss the superiority of ZnGa2O4 relative to TiO2 on the degradation of RhB, the proposed SLS growth and TMC process synthetic strategies may be of general significance in the preparation TiO2 and other types of photocatalysts.
In conclusion, we demonstrated the controlled synthesis of ZnGa2O4 nanorod arrays from hexagonal ZnO microdishes for the advanced application of photodegradation in water purification. The as-prepared ZnGa2O4 sample has highly self-supported, aligned 1-D nanostructures, where ZnGa2O4 nanorods with 50–80 nm diameter are vertically assembled into micro-architectures without extraneous supporting substrates. The ZnGa2O4 nanorod arrays showed much higher BET specific surface areas over the hexagonal ZnO microdishes. Due to favorable 1-D nanostructures and unique electronic band structures, the ZnGa2O4 photocatalyst exhibited a much higher photocatalytic activity relative to the hexagonal ZnO microdishes, in which the degradation rate of RhB can be 4.22 times as high as that of the ZnO photocatalyst. The findings suggest that this newly fashioned architecture of ZnGa2O4 could be used as a promising photocatalytic material for organic dye degradation.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (21443006, 21173088 and 21105030), Natural Science Foundation of Guangdong Province (S2013040015162), Guangdong Province and Chinese Academy of Sciences Strategic Cooperative Project (2012B090400003), Science and Technology Project of Maoming (2014006) and Doctor Startup Project of School (513086).Notes and references
- A. Alivisatos, J. Phys. Chem., 1996, 100, 13226–13239 CrossRef CAS.
- Z. Zhuang, Q. Peng and Y. Li, Chem. Soc. Rev., 2011, 40, 5492–5513 RSC.
- T. Zhai, L. Li, Y. Ma, M. Liao, X. Wang, X. Fang, J. Yao, Y. Bando and D. Golberg, Chem. Soc. Rev., 2011, 40, 2986–3004 RSC.
- A. Hochbaum and P. Yang, Chem. Rev., 2010, 110, 527–546 CrossRef CAS PubMed.
- (a) T. Wang, Z. Jiao, T. Chen, Y. Li, W. Ren, S. Lin, G. Lu, J. Ye and Y. Bi, Nanoscale, 2013, 5, 7552–7557 RSC; (b) M. Lv, D. Zheng, M. Ye, J. Xiao, W. Guo, Y. Lai, L. Sun, C. Lin and J. Zuo, Energy Environ. Sci., 2013, 6, 1615–1622 RSC.
- (a) L. Pan, H. Huang, C. Lim, Q. Hong, M. Tse and O. Tan, RSC Adv., 2013, 3, 3566–3571 RSC; (b) M. Sun, X. Ma, X. Chen, Y. Sun, X. Cui and Y. Lin, RSC Adv., 2014, 4, 1120–1127 RSC.
- M. Nischk, P. Mazierski, M. Gazda and A. Zaleska, Appl. Catal., B, 2014, 144, 674–685 CrossRef CAS PubMed.
- C. Zhang, H. Yu, Y. Li, L. Fu, Y. Gao, W. Song, Z. Shao and B. Yi, Nanoscale, 2013, 5, 6834–6841 RSC.
- (a) S. Xu and Z. Wang, Nano Res., 2011, 4, 1013–1098 CrossRef CAS; (b) C. Lai, X. Wang, Y. Zhao, H. Fong and Z. Zhu, RSC Adv., 2013, 3, 6640–6645 RSC.
- (a) L. Liu, J. Huang, L. Cao, J. Wu, J. Fei, H. Ouyang, F. Ma and C. Zhou, Mater. Lett., 2013, 95, 160–163 CrossRef CAS PubMed; (b) S. Yan, S. Ouyang, J. Gao, M. Yang, J. Feng, X. Fan, L. Wan, Z. Li, J. Ye, Y. Zhou and Z. Zou, Angew. Chem., 2010, 122, 6544–6548 CrossRef.
- (a) W. Zhang, J. Zhang, Z. Chen and T. Wang, Catal. Commun., 2009, 101, 781–1785 Search PubMed; (b) W. Zhang, J. Zhang, X. Lan, Z. Chen and T. Wang, Catal. Commun., 2010, 11, 1104–1108 CrossRef CAS PubMed.
- M. Zhong, Y. Li, T. Tokizono, M. Zheng, I. Yamada and J. Delaunay, J. Nanopart. Res., 2012, 14, 804–814 CrossRef.
- L. Tien, C. Tseng, Y. Chen and C. Ho, J. Alloys Compd., 2013, 555, 325–329 CrossRef CAS PubMed.
- (a) H. Yu and W. Buhro, Adv. Mater., 2003, 15, 416–419 CrossRef CAS; (b) J. Sun, L. Wang and W. Buhro, J. Am. Chem. Soc., 2008, 130, 7997–8005 CrossRef CAS PubMed.
- Y. Fang, X. Wen and S. Yang, Angew. Chem., 2006, 118, 4771–4774 CrossRef.
- A. Qin, X. Zhou, Y. Qiu, Y. Fang, C. Su and S. Yang, Adv. Mater., 2008, 207, 68–773 Search PubMed.
- S. Yang, C. Ge, Z. Liu, Y. Fang, Z. Li, D. Kuang and C. Su, RSC Adv., 2011, 1, 1691–1694 RSC.
- (a) L. Tian, H. Zou, J. Fu, X. Yang, Y. Wang, H. Guo, X. Fu, C. Liang, M. Wu, P. Shen and Q. Gao, Adv. Funct. Mater., 2010, 20, 617–623 CrossRef CAS; (b) J. Kim, B. Fang, M. Song and J. Yu, Chem. Mater., 2012, 24, 2256–2264 CrossRef CAS.
- X. Zhou, Y. Liu, X. Li, Q. Gao, X. Liu and Y. Fang, Chem. Commun., 2014, 50, 1070–1073 RSC.
- S. Sampath, D. Kanhere and R. Pandey, J. Phys.: Condens. Matter, 1999, 11, 3635 CrossRef CAS.
- S. Bae, J. Lee, H. Jung, J. Park and J. Ahn, J. Am. Chem. Soc., 2005, 127, 10802–10803 CrossRef CAS PubMed.
- G. Chen, M. Sun, Q. Wei, Y. Zhang, B. Zhu and B. Dua, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- J. Zhang, Z. Zhu, Y. Tang and X. Feng, J. Mater. Chem. A, 2013, 1, 3752–3756 CAS.
- B. Weng, M. Yang, N. Zhang and Y. Xu, J. Mater. Chem. A, 2014, 2, 9380–9389 CAS.
- J. Wang, C. An, J. Liu, G. Xi, W. Jiang, S. Wang and Q. Zhang, J. Mater. Chem. A, 2013, 1, 2827–2832 CAS.
- H. Li, S. Yin, Y. Wang, T. Sekino, S. W. Lee and T. Satoa, J. Mater. Chem. A, 2013, 1, 1123–1126 CAS.
- D. Zhang, J. Li, Q. Wang and Q. Wu, J. Mater. Chem. A, 2013, 1, 8622–8629 CAS.
- C. Pan, J. Xu, Y. Chen and Y. Zhu, Appl. Catal., B, 2012, 115–116, 314–319 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05863a |
| This journal is © The Royal Society of Chemistry 2014 |