Thiourea as an efficient organocatalyst for the transfer hydrogenation of 2-substituted quinoline derivatives†
Xiang Qiao,
Zhiguo Zhang*,
Zongbi Bao,
Baogen Su,
Huabin Xing,
Qiwei Yang and
Qilong Ren
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, Department of Chemical and Biological Engineering, Zhejiang University, Zheda Road 38, Hangzhou 310027, China. E-mail: zhiguo.zhang@zju.edu.cn; Fax: +86-571-87952375; Tel: +86-571-87951224
First published on 2nd September 2014
Abstract
This study reports on the first transfer hydrogenation of 2-substituted quinolines promoted by a thiourea catalyst with Hantzsch ester as the hydrogen source. A wide range of quinoline derivatives, including 2-alkylquinolines and 2-arylquinolines, were efficiently reduced to give 1,2,3,4-tetrahydroquinolines with good to excellent yields. The operational simplicities and conveniences of this reaction pathway render this protocol considerably promising in the preparation of biologically active tetrahydroquinoline derivatives.
2-Substituted-1,2,3,4-tetrahydroquinoline architectures are widely found in numerous biologically active natural products and pharmaceutical compounds.1 For example (Fig. 1), this framework is universal in many natural occurring alkaloids, i.e. angustureine, galipinine, cuspareine, etc.2 As far as chemotherapeutic potential is concerned, 2-substituted tetrahydropyridine derivatives have been also reported to possess a variety of biological activities such as antiarrhythmic (oxamniquine), high-density lipoprotein cholesterol-lowering (torcetrapib) and antibiotic (virantmycin) activities.3
Direct hydrogenation of quinoline derivatives, which are easily prepared from readily available starting materials,4 represents a concise approach to tetrahydroquinoline compounds.5 However, these methods are currently dominated by the use of precious metal catalysts in conjunction with molecular hydrogen, which are often carried out under relatively rigorous operation conditions, i.e. in air- and moisture-free as well as high-pressure resistant apparatus.6 From the viewpoint of operational simplicity, the development of an easily manageable synthetic protocol is therefore highly desirable.
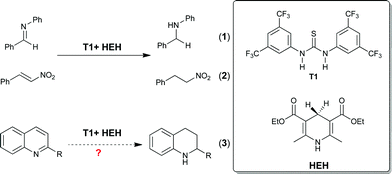
At the beginning of this century, the chemical transformations promoted by metal-free organic molecules started to shed light into the synthetic world and serve as an alternative method to metal- and biocatalysis.7 In this context, transfer hydrogenation of unsaturated compounds such as imines, olefins as well as carbonyls catalyzed by organocatalysts in combination with organic hydride donors has resulted in new paradigm in the reduction of unsaturated compounds.8 As one of the most widely used organic hydride donors, Hantzsch ester (HEH), since its advent,9 has been employed as a biomimetic mimics to NAD(P)H in a series of redox reactions.10 Recently, Rueping and other research groups have developed a number of Brønsted acid-catalyzed transfer hydrogenation of quinolines with HEH as reducing agent.11 Similar to well-developed Brønsted acid-based catalysts, thiourea derivatives have also been widely employed as efficient catalysts in a variety of chemical transformations.12 As part of our ongoing research interests in the development of H-bond mediated transfer hydrogenation methods, we have reported the reduction of aldimines (Fig. 2, eqn 1) and nitroolefins (Fig. 2, eqn 2) in the presence of privileged thiourea catalyst T1 (ref. 13) using HEH as the hydrogen source.14 Inspired by these observations, we reasoned that the interaction of thiourea T1 with the nitrogen atom contained in the quinoline through hydrogen bonding may dramatically reduce the barrier energy and consequently promote the tandem hydride transfer from HEH to the nitrogen-containing part of quinolines, thereby furnishing the tetrahydroquinolines (Fig. 2, eqn 3). Herein, in continuation to our early work, we describe the first example of thiourea as H-bond catalyst for the transfer hydrogenation of quinolines using HEH as the corresponding reductant.
Our initial study began with the investigation of the feasibility of our strategy. In the presence of 10 mol% of T1 and 2.2 equiv. of HEH, the transfer hydrogenation of 2-phenylquinoline was selected as the standard reaction for the optimization of reaction conditions. As shown in Table 1, only trace amount of product was observed when the reaction was performed at ambient temperature in CH2Cl2 (Table 1, entry 1). The reaction proceeded smoothly in toluene when the reaction temperature increased to 60 °C, giving the product in 70% conversion at 24 h (Table 1, entry 3). To our delight, the conversion can be further improved when increasing the amount of HEH to 3.0 equiv., whereby the slight excess of HEH is probably due to its partial oxidation at air. Note that the catalyst loading of T1 can be reduced to 3 mol% without obvious compromise of the conversion (Table 1, entry 6). The polarities of the solvents also had a significant effect on the reaction outcome. When polar solvents such as EtOAc, MeCN, DMF, MeOH, THF were used (Table 1, entries 8–12), the conversion of this reaction decreased dramatically, which is in agreement with the deleterious effect of polar solvents to H-bond formation during the transition state. Apolar solvents generally suitable for this catalytic pathway, among others, toluene has been proved to be the best reaction media. Control experiments revealed that no reaction happened in the absence of T1 catalyst (Table 1, entry 13).
| Entrya | T1 (mol%) | HEH (equiv.) | Solvent | T (°C) | t (h) | Conv.b (%) |
|---|---|---|---|---|---|---|
| a All reactions were performed using 2-phenylquinoline (0.20 mmol) and HEH in 1 ml of solvents in the presence of T1 as the catalyst.b Determined by GC analysis with tridecane as the internal standard. | ||||||
| 1 | 10 | 2.2 | CH2Cl2 | r.t. | 72 | Trace |
| 2 | 10 | 2.2 | CH2Cl2 | 40 | 24 | 15 |
| 3 | 10 | 2.2 | Toluene | 60 | 24 | 70 |
| 4 | 10 | 3.0 | Toluene | 60 | 24 | 97 |
| 5 | 5 | 3.0 | Toluene | 60 | 24 | 97 |
| 6 | 3 | 3.0 | Toluene | 60 | 24 | 90 |
| 7 | 3 | 3.0 | CHCl3 | 60 | 24 | 75 |
| 8 | 3 | 3.0 | EtOAc | 60 | 24 | 48 |
| 9 | 3 | 3.0 | MeCN | 60 | 24 | 49 |
| 10 | 3 | 3.0 | DMF | 60 | 24 | 3 |
| 11 | 3 | 3.0 | MeOH | 60 | 24 | 23 |
| 12 | 3 | 3.0 | THF | 60 | 24 | 7 |
| 13 | 0 | 3.0 | Toluene | 60 | 24 | 0 |
Under the optimal reaction conditions, the substrate scope of this T1-catalyzed transfer hydrogenation of quinolines was examined. As shown in Table 2, various substituents on the phenyl ring of 2-substituted quinolines did not have much influence on the catalytic efficiency. Both electron-donating groups (Me, OMe, Ph, etc.) (Table 2, entries 2–6) and the electron-withdrawing groups (F, Cl, Br) (Table 2, entries 7–9) are well applicable to this protocol, furnishing the corresponding tetrahydroquinolines in good to excellent yields. Moreover, it is worthy of noting that 2-alkyl substituted quinolines also proceed smoothly, affording the corresponding products with yield of 97% and 77% (Table 2, entries 10–11), respectively.
| Entrya | R | Time (h) | Yieldb (%) |
|---|---|---|---|
| a All reactions were performed using quinoline (0.20 mmol) and HEH (0.60 mmol) at 60 °C in 1 ml of toluene in the presence of 3 mol% of T1.b Isolated yield after chromatography. | |||
| 1 | Phenyl | 24 | 90 |
| 2 | 4-Methylphenyl | 18 | 95 |
| 3 | 2,4-Dimethylphenyl | 24 | 77 |
| 4 | 4-Isopropylphenyl | 24 | 79 |
| 5 | 4-Methoxyphenyl | 36 | 96 |
| 6 | 1,1′-Biphenyl-4-yl | 24 | 87 |
| 7 | 2-Fluorophenyl | 24 | 69 |
| 8 | 4-Chlorophenyl | 24 | 82 |
| 9 | 4-Bromophenyl | 48 | 90 |
| 10 | n-Butyl | 36 | 97 |
| 11 | Isopropyl | 36 | 77 |
Conclusions
In summary, we have developed a thiourea-catalyzed transfer hydrogenation of 2-substituted quinolines with HEH as the hydrogen source, which gives direct access to a variety of 2-aryl- and 2-alkyl-substituted tetrahydroquinolines with good to excellent yields. This organocatalytic process not only further expands the application scope of thiourea T1, but also demonstrates for the first time that the hydride transfer from HEH to quinolines can be successfully promoted via H-bond interaction. The mild reaction conditions as well as the relatively low catalyst loading make this transformation an attractive approach to tetrahydroquinoline derivatives. Further work will be directed toward the application of chiral thiourea catalyzed enantioselective transfer hydrogenation of quinolines to enantioenriched tetrahydroquinoline derivatives as well as other heteroaromatic systems.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21376212, 21222601), Doctoral Fund of Ministry of Education of China (no. 20120101120107) and the Natural Science Foundation of Zhejiang Province, China (no. LY13B060001).Notes and references
- For review, see: A. R. Katritzky, S. Rachwal and B. Rachwal, Tetrahedron, 1996, 52, 15031–15070 CrossRef CAS.
- I. Jacquemond-Collet, F. Benoit-Vical, Mustofa, A. Valentin, E. Stanislas, M. Mallie and I. Fouraste, Planta Med., 2002, 68, 68–69 CrossRef CAS PubMed.
- (a) P. G. Fallon and M. J. Doenhoff, Am. J. Trop. Med. Hyg., 1994, 51, 83–88 CAS; (b) A. Nakagawa, Y. Iwai, H. Hashimoto, N. Miyazaki, R. Oiwa, Y. Takahashi, A. Hirano, N. Shibukawa, Y. Kojima and S. Omura, J. Antibiot., 1981, 34, 1408–1415 CrossRef CAS; (c) M. E. Brousseau, E. J. Schaefer, M. L. Wolfe, L. T. Bloedon, A. G. Digenio, R. W. Clark, J. P. Mancuso and D. J. Rader, N. Engl. J. Med., 2004, 350, 1505–1515 CrossRef CAS PubMed.
- S. M. Prajapati, K. D. Patel, R. H. Vekariya, S. N. Panchal and H. D. Patel, RSC Adv., 2014, 4, 24463 RSC.
- For a comprehensive review, see: D. S. Wang, Q. A. Chen, S. M. Lu and Y. G. Zhou, Chem. Rev., 2012, 112, 2557–2590 CrossRef CAS PubMed.
- For selected examples, see: (a) W. B. Wang, S. M. Lu, P. Y. Yang, X. W. Han and Y. G. Zhou, J. Am. Chem. Soc., 2003, 125, 10536–10537 CrossRef CAS PubMed; (b) F. Glorius, Org. Biomol. Chem., 2005, 3, 4171–4175 RSC; (c) K. H. Lam, L. Xu, L. Feng, Q.-H. Fan, F. L. Lam, W.-h. Lo and A. S. C. Chan, Adv. Synth. Catal., 2005, 347, 1755–1758 CrossRef CAS PubMed; (d) D. W. Wang, W. Zeng and Y. G. Zhou, Tetrahedron: Asymmetry, 2007, 18, 1103–1107 CrossRef CAS PubMed; (e) N. Mrsic, L. Lefort, J. A. F. Boogers, A. J. Minnaard, B. L. Feringa and J. G. de Vries, Adv. Synth. Catal., 2008, 350, 1081–1089 CrossRef CAS PubMed; (f) S.-M. Lu and C. Bolm, Adv. Synth. Catal., 2008, 350, 1101–1105 CrossRef CAS PubMed; (g) D. W. Wang, X. B. Wang, D. S. Wang, S. M. Lu, Y. G. Zhou and Y. X. Li, J. Org. Chem., 2009, 74, 2780–2787 CrossRef CAS PubMed; (h) H. Tadaoka, D. Cartigny, T. Nagano, T. Gosavi, T. Ayad, J.-P. Genêt, T. Ohshima, V. Ratovelomanana-Vidal and K. Mashima, Chem.–Eur. J., 2009, 15, 9990–9994 CrossRef CAS PubMed; (i) Z. J. Wang, H. F. Zhou, T. L. Wang, Y. M. He and Q. H. Fan, Green Chem., 2009, 11, 767–769 RSC; (j) W. Tang, Y. Sun, L. Xu, T. Wang, Q. Fan, K. H. Lam and A. S. Chan, Org. Biomol. Chem., 2010, 8, 3464–3471 RSC; (k) T. Wang, L.-G. Zhuo, Z. Li, F. Chen, Z. Ding, Y. He, Q. H. Fan, J. Xiang, Z. X. Yu and A. S. C. Chan, J. Am. Chem. Soc., 2011, 133, 9878–9891 CrossRef CAS PubMed; (l) S. Urban, N. Ortega and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 3803–3806 CrossRef CAS PubMed; (m) A. M. Maj, I. Suisse, C. Hardouin and F. Agbossou-Niedercorn, Tetrahedron, 2013, 69, 9322–9328 CrossRef CAS PubMed.
- For representative books, see: (a) B. List, Asymmetric organocatalysis, Springer, Heidelberg, 2010 Search PubMed; (b) P. I. Dalko, Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications, Wiley-VCH, Weinheim, 2013 Search PubMed.
- For representative reviews, see: (a) S. G. Ouellet, A. M. Walji and D. W. C. Macmillan, Acc. Chem. Res., 2007, 40, 1327–1339 CrossRef CAS PubMed; (b) A. V. Malkov, K. Vrankova, S. Stoncius and P. Kocovsky, J. Org. Chem., 2009, 74, 5839–5849 CrossRef CAS PubMed.
- A. Hantzsch, Ber. Dtsch. Chem. Ges., 1881, 14, 1637–1638 CrossRef PubMed.
- For reviews, see: (a) S. L. You, Chem.–Asian J., 2007, 2, 820–827 CrossRef CAS PubMed; (b) M. Rueping, J. Dufour and F. R. Schoepke, Green Chem., 2011, 13, 1084–1105 RSC; (c) M. Rueping, E. Sugiono and F. R. Schoepke, Synlett, 2010, 852–865 CrossRef CAS PubMed; (d) C. Zheng and S. L. You, Chem. Soc. Rev., 2012, 41, 2498–2518 RSC.
- (a) M. Rueping, T. Theissmann and A. P. Antonchick, Synlett, 2006, 1071–1074 CrossRef CAS; (b) M. Rueping, A. P. Antonchick and T. Theissmann, Angew. Chem., Int. Ed., 2006, 45, 3683–3686 CrossRef CAS PubMed; (c) Q. S. Guo, D. M. Du and J. Xu, Angew. Chem., Int. Ed., 2008, 47, 759–762 CrossRef CAS PubMed; (d) M. Rueping and T. Theissmann, Chem. Sci., 2010, 1, 473–476 RSC; (e) L. Ren, T. Lei, J. X. Ye and L. Z. Gong, Angew. Chem., Int. Ed., 2012, 51, 771–774 CrossRef CAS PubMed; (f) D. S. Kundu, J. Schmidt, C. Bleschke, A. Thomas and S. Blechert, Angew. Chem., Int. Ed., 2012, 51, 5456–5459 CrossRef CAS PubMed; (g) J. Stemper, K. Isaac, V. Duret, P. Retailleau, A. Voituriez, J. F. Betzer and A. Marinetti, Chem. Commun., 2013, 49, 6084–6086 RSC.
- For selected reviews and examples, see: (a) T. Akiyama, Chem. Rev., 2007, 107, 5744–5758 CrossRef CAS PubMed; (b) T. Akiyama, J. Itoh and K. Fuchibe, Adv. Synth. Catal., 2006, 348, 999–1010 CrossRef CAS PubMed; (c) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713–5743 CrossRef CAS PubMed; (d) X. Yu and W. Wang, Chem.–Asian J., 2008, 3, 516–532 CrossRef CAS PubMed; (e) Z. Zhang and P. R. Schreiner, Chem. Soc. Rev., 2009, 38, 1187–1198 RSC; (f) S. J. Connon, Chem. Commun., 2008, 2499–2510 RSC; (g) G. D. Joly and E. N. Jacobsen, J. Am. Chem. Soc., 2004, 126, 4102–4103 CrossRef CAS PubMed; (h) N. J. Martin, L. Ozores and B. List, J. Am. Chem. Soc., 2007, 129, 8976–8977 CrossRef CAS PubMed; (i) M. Rueping, A. Kuenkel and R. Fröhlich, Chem.–Eur. J., 2010, 16, 4173–4176 CrossRef CAS PubMed; (j) J. F. Schneider, M. B. Lauber, V. Muhr, D. Kratzer and J. Paradies, Org. Biomol. Chem., 2011, 9, 4323–4327 RSC; (k) M. Kotke and P. Schreiner, in Hydrogen Bonding in Organic Synthesis, ed. P. M. Pihko, Wiley-VCH, Weinheim, 2009, pp. 141–351 Search PubMed.
- For a comprehensive review on T1 catalysis, see: Z. G. Zhang, Z. B. Bao and H. B. Xing, Org. Biomol. Chem., 2014, 12, 3151–3162 CAS.
- (a) Z. G. Zhang and P. R. Schreiner, Synlett, 2007, 1455–1457 CAS; (b) Z. G. Zhang and P. R. Schreiner, Synthesis, 2007, 2559–2564 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05854b |
| This journal is © The Royal Society of Chemistry 2014 |