Electrochemical sensor based on overoxidized dopamine polymer and 3,4,9,10-perylenetetracarboxylic acid for simultaneous determination of ascorbic acid, dopamine, uric acid, xanthine and hypoxanthine†
Abstract
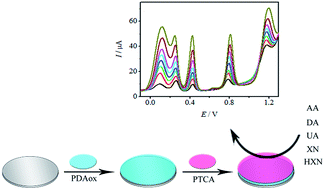
A novel electrode based on 3,4,9,10-perylenetetracarboxylic acid (PTCA) and overoxidized dopamine polymer (PDAox) was developed for the simultaneous determination of ascorbic acid (AA), dopamine (DA), uric acid (UA), xanthine (XN) and hypoxanthine (HXN). The developed sensors exhibited an excellent catalytic activity, high sensitivity and good selectivity toward the oxidation of AA, DA, UA, XN and HXN. Scanning electron microscopy (SEM), cyclic voltammetry (CV), different pulse voltammetry (DPV) and electrochemical impedance spectroscopy (EIS) were employed to characterize the sensor. The peak separations between AA–DA, DA–UA, UA–XN and XN–HXN were large, up to 0.15, 0.18, 0.37 and 0.4 V, respectively. The calibration curves for AA, DA, UA, XN and HXN were obtained in the ranges of 76 μM to 3.9 mM, 0.60 to 253 μM, 1.8 to 238 μM, 5.1 to 289 μM and 3.8 to 293 μM with detection limits (S/N = 3) of 25.3 μM, 0.20 μM, 0.60 μM, 1.7 μM and 1.3 μM, respectively. The integration of PDAox and PTCA in the sensor opens up a facile and promising method for the simultaneous determination of above five substances.

 Please wait while we load your content...
Please wait while we load your content...