Removal of refractory sulfur and aromatic compounds from straight run gas oil using solvent extraction
Sunil Kumarab,
Vimal Chandra Srivastava*a,
S. M. Nanotib,
B. R. Nautiyalb and
Siyaramb
aDepartment of Chemical Engineering, Indian Institute of Technology Roorkee, Roorkee 247667, Uttarakhand, India. E-mail: vimalcsr@yahoo.co.in; sunilk@iip.res.in; Fax: +91-1332-276535; Tel: +91-1332-285889
bIndian Institute of Petroleum, Dehradun 248005, Uttarakhand, India. E-mail: smnanoti@iip.res.in; bnauti@iip.res.in; siyaram@iip.res.in
First published on 6th August 2014
Abstract
In the present study, extraction of sulfur and polyaromatic impurities from actual straight run gas oil (SRGO) containing 1.3 wt% sulfur was studied using various solvents such as acetonitrile, N,N-dimethylformamide, furfural, N,N-dimethylacetamide and dimethyl sulfoxide. Effects of water as antisolvent, extraction conditions and types of extraction operation (batch, single and multi-stage and continuous) have been studied. Performance of solvent extraction process, which is governed by the degree of sulfur removal (Dsr) and yield of extracted SRGO (ESRGO), has been evaluated in terms of a performance factor (Pf,α), which has been defined in terms of weight factor (0 < α < 1) as: Pf,α = αDsr + (1 − α) yield. DMF was found to be a better solvent in terms of Pf,α and regeneration ability. The possibility of utilization of extract as carbon black feed stock (CBFS) has also been discussed based upon the calculated bureau of mines correlation index (BMCI) values.
Introduction
Gas oil is one of the most commonly used transportation fuel and contributes significant harmful emission of NOx, SOx, hydrocarbons and particulate matter to the environment. This leads to serious environmental and health concerns, such as smog, global warming, water pollution, acid rain, cancer and neurotoxicity.1–5 The quantity of these emissions significantly depends on the concentration of sulfur, nitrogen and aromatic compounds in the gas oil being used.5 Environmental regulations have been implemented across the globe to limit the sulfur and aromatic content of gas oil for improving the air quality.6,7Currently, refining industry is facing a serious challenge of meeting the increasing demand for gas oil along with the required stringent specifications. This challenge shall become more serious in future due to the necessity of processing the sour and heavy crudes due to their increased availability. Hydrodesulfurization is the most developed and commonly used process in refineries for removing sulfur aromatic compounds from gas oil.
The cost of clean gas oil production from sour crude using conventional hydrotreatment method is increasing and has become drastically high due to the requirement of severe operating conditions, more amount of hydrogen, noble metal based very active and expensive catalysts and huge revamp and capital depreciation cost. Refiners are looking forward to adopt a methodology to reduce the production cost of clean diesel to address the challenge of reduced profit margin in refining. Selective solvent extraction appears to be an economic solution to modify high straight run gas oil (SRGO) by removing maximum amount of refractory sulfur prior to further treating it by conventional hydrotreatment method thereby reducing the sulfur removal load and the cost of hydrotreatment or other alternative methods.
There are many methods for the removal of sulfur and aromatic compounds, such as extraction, oxidative-extraction, adsorption and oxidative-adsorption, which can be used either individually or as complementary with hydrotreatment to produce ultra clean gas oil from straight run gas oil economically. Desulfurization using ionic liquids is widely being researched all over the world.8,9
Extraction with selective solvent is well proven and widely adapted process in modern refining industries for production/removal of aromatic hydrocarbons from various hydrocarbon streams.5,10 There are some studies which reported the removal of sulfur and heteroatomic sulphur, and nitrogen compounds from boiling range hydrocarbon stream of gas oil using the selective solvent extraction process.11–18 These studies evaluated the performance of various solvents for sulfur and aromatics removal from gas oil using single stage and multiple stage batch extraction systems. However, to our knowledge there are no reported studies on continuous counter-current extraction column for extractive desulfurization. Similarly, application of antisolvent like water in sulfur extraction process is reported in very few studies.14,15 Extraction process is a trade-off between the degree of sulfur removal (Dsr) and yield of extracted SRGO (ESRGO). No study is reported in the literature to the best of the authors' knowledge for the study of the performance of an extraction process in terms of a factor which combines both Dsr and yield.
Considering the above discussions, the present study includes the evaluation of the performance of industrially proven and viable solvents for the removal of sulfur compounds from SRGO containing high sulfur content (1.3 wt%). The effect of extraction temperature, solvent to feed ratio, antisolvent concentration and number of stages (during batch operation) on the degree of sulfur and aromatics removal and yield were evaluated for the batch and continuous counter-current extraction systems. A strategy on reutilization of the extract from industrial point of view has also been suggested.
Theory
Liquid–liquid solvent extraction is based on the principle of difference in the solubility of solute compounds in a solvent. The degree of solubility of solutes in solvent depends upon their chemical nature. Solvent extraction process involves the removal of impurities via scrubbing the hydrocarbon stream by solvent and recovery of the solvent from the scrubbed impurities for its reuse. The major challenge in the solvent extraction is to tackle the problem of the loss of desired hydrocarbons with the removed impurities. This loss depends on the capacity and selectivity of the solvent which can be adjusted by addition of co/antisolvent and changing the extraction temperature.A number of solvents have been reported for the extraction of sulfur and aromatic compounds from gas oil.11–18 However, from techno-economic point of view, the selection of the solvent depends on physico-chemical characteristics of the solvent and feedstock, e.g. boiling point/boiling range, density, viscosity, melting point, miscibility, the capacity and selectivity of solvent. The desirable features for extraction solvent have been summarized in literature.19–24
The following physical properties can be used as preliminary tools to screen the solvents: (1) sufficient difference in the density between solvent and feed for allowing two phase formation and avoiding the flooding condition during extraction; (2) sufficient boiling point difference between solvent and feed to facilitate easy recovery of solvent for its reuse; (3) high thermal and chemical stability of solvent to avoid loss due to degradation; (4) low melting point to avoid steam tracing; (5) low viscosity for the high rate of mass transfer; (6) no zoetrope formation with components in feed to facilitate easy recovery; (7) non-toxicity for safe operation; and (8) noncorrosive to reduce capital investment. Moreover, the selected solvent should further be evaluated for their high capacity for solutes to reduce the required solvent to feed (S/F) ratio and high selectivity to reduce the height of extractor and improve the quality of extract, and to increase the yield of raffinate. The capacity and selectivity of solvent can be adjusted by changing the quantity of co- and anti-solvent in the main solvent and the extraction temperature.
The usefulness of a solvent in liquid–liquid extraction can be represented by the extraction factor for sulfur, sulfur distribution coefficient, yield of extracted gas oil, degree of sulfur removal, and performance factor.
Extraction factor is used to represent the capacity of a solvent. Extraction factor (εs) for single stage solvent extraction is defined as.25
 | (1) |
The distribution coefficient of solute (Ks) is defined as the ratio of composition for solute in the extract phase to that in the raffinate phase and is represented as follows:
| Ks = ys/xs | (2) |
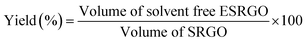 | (3) |
The capacity of a solvent is a measure of its ability to dissolve the hydrocarbon. Considering this volumetric yield of ESRGO can also be used to represent the capacity of solvent.
Material and component balance equations which are required to estimate the unknown value of variable in raffinate/extract phase are defined as follows:
| F = R + E | (4) |
| xf,iF = xr,iR + xe,iE | (5) |
Degree of sulfur removal (Dsr) used in this study was estimated using the following expression:
 | (6) |
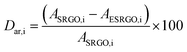 | (7) |
Since capacity and selectivity of solvent show opposite trends, it can be understood that the yield of ESRGO would decrease with the increase in the degree of removal of sulfur and aromatic compounds. However, from economic point of view of a process, it is desirable to obtain the maximum yield of ESRGO with maximum removal of sulfur and poly aromatic compounds. To combine the effect of these two important parameters in a single factor, performance factor (Pf) of solvent, first time used in this study, is defined as follows:
| Pf,α = αDsr + (1 − α) × Yield(%) | (8) |
Experimental
Materials
Straight run gas oil (SRGO) was obtained from an Indian refinery. Physicochemical properties of SRGO are given in Table 1. Acetonitrile (AcN: 99.5%+, MERCK), N,N-dimethylformamide (DMF: 99.5%, MERCK), furfural (FF: 98%: SD Fines), N,N-dimethylacetamide (DMA: 99.5%+, MERCK) and dimethyl sulfoxide (DMSO: 99.8%, MERCK) were used as extraction solvents. All compounds mentioned above were used without any pretreatment except furfural. Furfural was distilled prior to use as a solvent.| Parameter | Value |
|---|---|
| Total sulfur (wt%) | 1.3 |
| Non aromatics | 66.8 |
| Mono-aromatics | 18.8 |
| Di-aromatics | 8.2 |
| Poly-aromatics | 6.2 |
| Refractive index nD20 | 1.4762 |
| Density at 20 °C (kg m−3) | 853.24 |
| Kinematic viscosity at 70 °C (cSt) | 2.17 |
| Kinematic viscosity at 100 °C (cSt) | 1.44 |
| ASTM D-86 | |
|---|---|
| Volume% | Temperature, °C |
| IBP | 222.1 |
| 5 | 244.5 |
| 10 | 251.2 |
| 20 | 259.5 |
| 30 | 266.8 |
| 40 | 276.1 |
| 50 | 287.2 |
| 60 | 300.3 |
| 70 | 315 |
| 80 | 332.1 |
| 90 | 351.8 |
| 95 | 369.7 |
| FBP | 380.9 |
| Distillate | 97.0 |
| Residue | 2.5 |
| Lighter | 0.5 |
Methods of analysis
Density was determined using DE45 densitometer manufactured from Metller Toledo, Japan at temperature of 20 °C. Refractive index was determined using Abbe Refractometer RE45 at 20 °C. Total sulfur content of SRGO and ESRGO were measured by X-ray fluorescence (XRF) method by using ASOMA ED XRF analyzer Spectro Phoenix II make. ASTM D86 method was used for determining the boiling range of gas oil. Ultraviolet (UV) spectrophotometry was used for the estimation of mono, di and poly aromatics content of SRGO and ESRGO.Apparatus and procedure
Results and discussion
Batch equilibrium extraction
The results (Table 2) clearly indicate that the sulfur removal and yield of ESRGO using solvent extraction strongly depends on the type of solvent used. Percent sulfur removal using DMF, DMA and furfural solvents are much higher than AcN and DMSO. Among DMF, DMA and furfural, DMA removes maximum sulfur. However, yield values for DMF, DMA and furfural solvents are lower than AcN and DMSO. This indicates that there is a trade-off between sulfur removal and yield value. From the economic point of view of the process, maximum sulfur removal with maximum yield value is desirable.
| ACN | DMF | DMA | Furfural | DMSO | |
|---|---|---|---|---|---|
| Raffinate properties | |||||
| Refractive index@20 °C | 1.469 | 1.4635 | 1.4623 | 1.4657 | 1.4701 |
| Density@20 °C, g ml−1 | 0.84304 | 0.83521 | 0.83280 | 0.83468 | 0.84255 |
| Sulfur in ESRGO (%) | 1.18 | 0.81 | 0.76 | 0.8 | 1.03 |
| Calculate responses | |||||
| Gas oil yield % | 87.5 | 81 | 72.5 | 82.5 | 88.5 |
| Extraction factor | 0.26 | 0.98 | 1.36 | 0.97 | 0.43 |
| Distribution coefficient | 0.22 | 0.62 | 0.73 | 0.53 | 0.28 |
| Sulfur removal (%) | 9.2 | 37.7 | 41.5 | 38.5 | 20.8 |
| Performance factor | 48.4 | 59.3 | 57 | 60.5 | 54.6 |
Considering this, the performance factor (Pf) for each solvent was estimated using eqn (8) and weight factor (α) = 0.5, thus giving equal importance to the yield and Dsr. The values of Pf are given in Table 3. It is observed that when the sulfur removal and yield were assigned the same value of α, values of Pf follow the order: furfural > DMF > DMA > DMSO > AcN.
The extraction factor and distribution coefficient follow the order: DMA > DMF > furfural > DMSO > AcN. Although the values of extraction factor for DMF and furfural are comparable, there is significant difference in their distribution coefficients. It may be attributed to the difference in the density of the solvents.
The effect of the value of α (in the range of 0.3–0.9) on the Pf value for each solvent is shown in Fig. 1. It is clear that Pf values for DMF and furfural solvents are very close to each other and they decrease over the whole range of α. For AcN and DMSO, the values of Pf are always much less as compared to other solvents at all values of α. Pf values of DMF are higher than that of DMA for α < 0.7, however, for α ≥ 0.7 Pf values of DMA become higher than that of DMF. Pf values for furfural are slightly higher than that of DMF, however, its value becomes lower than that of DMA for α ≥ 0.8. Therefore, DMF, furfural and DMA appear to be comparable solvents in terms of Pf for the whole range of α.
 | ||
| Fig. 1 Effect of weight (α) on solvent's performance factor (Pf,α) during the extractive desulfurization of SRGO by various solvents. | ||
However, considering the low oxidative and thermal stability of furfural as reported in literature,5 although DMF and DMA have lower values of extraction factor and distribution coefficient, they were selected as solvents for further study.
 | ||
| Fig. 2 Effect of extraction temperature on volume yield%, degree of sulfur removal (Dsr) and solvent's performance factor (Pf,α) for solvent to feed ratio of 1. | ||
Water behaves as an antisolvent during extraction process as it decreases the solubility of hydrocarbons in solvents. Concentration of antisolvent in the main solvent can change the performance of solvent.15 Considering this, extraction of SRGO with DMF and DMA solvents was carried out by varying the water content in the range of 0–3.0 volume% at 65 °C to understand the impact of the amount of water on Dsr and Pf of DMF and DMA. Results are shown in Fig. 3. As expected, an increase in water amount in the solvent, increased the ESRGO yield, however, it decreased the degree of sulfur removal for both the solvents. However, the extent of change in Pf,0.5 for DMA solvent was slightly higher than that of DMA with increasing water amount in solvent.
 | ||
| Fig. 3 Effect of anti-solvent (water) concentration on volume yield%, degree of sulfur removal (Dsr) and performance factor (Pf,α) for solvent to feed ratio = 1.0 and Temperature = 65 °C. | ||
The Pf values of DMA solvent are lower than that of DMF over the whole range of studied temperature and water amount. It can be inferred that when the degree of sulfur removal is more important than the yield, it is better to do the extraction at higher temperature and lower water amount and vice versa.
It should be noted that Pf values for DMF are higher than the DMA values for α ≤ 0.7. Considering the significance of ESRGO yield and the degree of sulfur removal in the economic point of view of the extraction process, DMF can be considered as more efficient than DMA. Moreover, the boiling points of DMF and DMA are 153 and 165 °C, respectively. Therefore, it is easier to recover DMF from the extract using distillation. In brief, considering all the above points, DMF can be considered as a better solvent than DMA, therefore, it was selected for further studies.
| Parameter | 1st stage | 2nd stage | 3rd stage |
|---|---|---|---|
| Stage wise volume yield (%) | 75.6 | 76.5 | 78.1 |
| Cumulative volume yield (%) | 75.6 | 57.8 | 45.2 |
| Sulfur in ESRGO | 0.74 | 0.53 | 0.36 |
| Dsr (%) | 43.1 | 28.4 | 32.1 |
| Cumulative Dsr (%) | 43.1 | 59.2 | 72.3 |
| Performance factor (Pf) | 59.3 | 52.4 | 55.1 |
Continuous counter current extraction
| Parameters | SRGO | B-ESRGO-55T-0W | C-SRGO-55T-0W | C-ESRGO-45T-0W | C-ESRGO-45T-3W | C-ESRGO-45T-5W |
|---|---|---|---|---|---|---|
| Experimental Conditions | ||||||
| Batch or continuous | Batch | Continuous | Continuous | Continuous | Continuous | |
| Extraction temp. (°C) | — | 55 | 55 | 45 | 45 | 45 |
| Water content (vol%) | — | 0 | 0 | 0 | 3 | 5 |
| Experimental results | ||||||
| Density at 20 °C (g ml−1) | 853.24 | 836.60 | 824.10 | 829.03 | 829.80 | 833.95 |
| Volume yield (%) | — | 79.1 | 74.3 | 78.1 | 84.5 | 86.7 |
| Sulfur (wt%) | 1.3 | 0.79 | 0.37 | 0.46 | 0.65 | 0.78 |
| Non-aromatics (wt%) | 71.3 | 80.9 | 89.4 | 88.4 | 85.6 | 83.5 |
| Mono-aromatics (wt%) | 16.8 | 12.5 | 8.7 | 8.9 | 9.6 | 10.5 |
| Di-aromatics (wt%) | 7.2 | 4.4 | 1.3 | 1.9 | 3.1 | 3.9 |
| Poly-aromatics (wt%) | 4.7 | 2.2 | 0.6 | 0.8 | 1.7 | 2.1 |
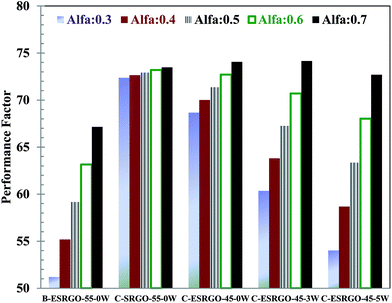
| Performance factor (Pf) | 31.0 | 53.2 | 50.5 | 42.3 | 34.7 | |
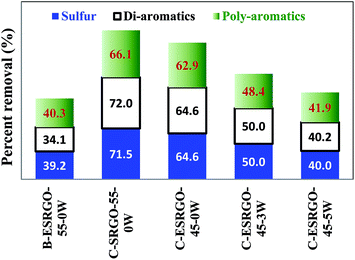 | ||
| Fig. 5 Comparative analysis of degree of sulfur, di-aromatics and poly-aromatics removal during batch and continuous extraction using DMF as solvent under various experimental conditions. | ||
It may be seen from Table 4 and Fig. 5 that the percent removal of these impurities is significantly greater by continuous extraction than batch extraction. It is attributed to the availability of more than one equilibrium stage in the column and increased concentration gradient across the counter current extraction column. The degree of removal of these impurities and the yield of ESRGO increased with increase in temperature and decrease in water content in solvent. The removal of undesired compounds followed the order: poly-aromatics > di-aromatic > sulfur > mono-aromatics. Removal of these compounds (which have very low cetane number) would significantly increase the cetane value of ESRGO.
It will also facilitate the easier deep desulfurization of gas oil in hydrotreater under less severe operating conditions due to the removal of refractory sulfur compounds and poly-aromatics responsible for slowing down the hydrotreatment reaction.26
Results suggest that the maximum extraction temperature and zero percent water concentration are desired for the maximum removal of these impurities. However, the yield of valuable ESRGO decreases with an increase in temperature and a decreasing water content.
Pf values for each case were estimated with different α values to understand the overall impact of extraction temperature and water amount on solvent extraction process. The results are shown in Fig. 6. It may be seen that the Pf,α value is significantly higher for the continuous extraction process in comparison to the batch extraction, irrespective of the value of α. For C-ESRGO-55T-0W case (extraction temperature = 55 and zero water content), the difference in Pf values is the least because the sulfur removal and yield number are close to each other. It may be clearly seen that the decrease in the extraction temperature spreads the Pf values i.e. there is large variation in Pf values for various α values (Pf,0.3 = 68.7 to Pf,0.7 = 74.1). Whereas, an increase in water content increases the spread of Pf values. For C-ESRGO-45T-3W case (extraction temperature = 45 and water content = 3 vol%), Pf,0.3 and Pf,0.7 values were 60.4 and 74.2, respectively. Further increase in water content for C-ESRGO-45T-5W case, increased the spread of the Pf values (Pf,0.3 = 54.0 to Pf,0.7 = 72.7). Overall for α = 0.7, there is marginal effect because of the variation of extraction temperature, however for α = 0.5, Pf values decreases with an increase in water content and decrease in temperature.
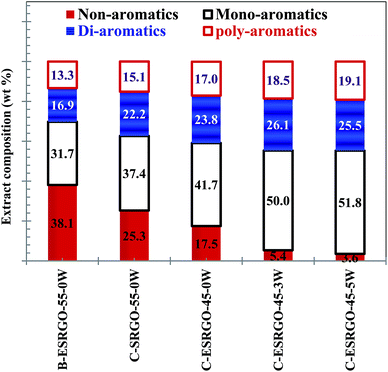 | ||
| Fig. 7 Composition of extract phase obtained during batch and continuous extraction using DMF as solvent under various experimental conditions. | ||
The FCC and delayed coker are processes where carbon is rejected to meet the requirement of hydrogen in distillate products. The metal content, Conradson carbon residue (CCR) and viscosity in extract stream obtained after continuous extraction would be very low and hydrogen to carbon (C/H) ratio would be significantly higher in comparison to vacuum residue (VR), thermal tar, lube extracts, pyrolysis tar and pitch streams which are used as feed in DCU. Therefore, the blending of extract stream with VR and pitch stream will not only increase the yield of distillates, which is inversely dependent on the CCR and proportionally dependent on C/H, but will also improve the quality of coke.27
Considering its utilization as a CBFS, bureau of mines correlation index (BMCI) value, which is indication of quality of black carbon feed stock for extract products, was estimated using the following correlation:28
BMCI = 473.7Sg − 456.8 + (48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460/Tb) 460/Tb)
| (9) |
The values of estimated BMCI values along with the estimated Sg of extract streams are tabulated in Table 5. Results indicate that BMCI value increases with decrease in extraction temperature and increase in water content in solvent as the selectivity of solvent for aromatics increases with respect to increase in paraffin compounds (Fig. 7). As we know, higher the BMCI, better the quality of CBFS; the solvent extraction should be carried out with a solvent containing significant amount of water. However, the increasing trend of the density of extract stream with decrease in temperature and water content also suggests decrease in the C/H ratio of extract. Therefore, there is a possibility of adjusting the operating conditions of the extraction unit considering the requirement of further downstream operation to be used for raffinate and extract stream processing. For example, temperature and S/F can be adjusted to the higher side with zero percent water in solvent to maximize the recovery of sulfur compounds to debottleneck the hydrotreatment unit to be used for raffinate processing in order to bring the sulfur to ppm level. While, the water content can be increased to increase the aromatic concentration in extract streams so that it can be used as a CBFS feed-stock and to increase the yield of ESRGO. Therefore, operating conditions of extraction is to be adjusted depending on the further considered process/application for extract and raffinate streams. It may be noted that the BMCI values obtained for the extracts are in the range of CBFS, which are being marketed by various refineries in India.29,30
| Parameter | B-ESRGO-55-0W | C-SRGO-55-0W | C-ESRGO-45-0W | C-ESRGO-45-3W | C-ESRGO-45-5W |
|---|---|---|---|---|---|
| Density @ 20 °C | 0.9162 | 0.9375 | 0.9396 | 0.9810 | 0.9853 |
| Density @ 15.5 °C | 0.9194 | 0.9407 | 0.9428 | 0.9842 | 0.9885 |
| Specific gravity @ 15.5 | 0.9199 | 0.9412 | 0.9433 | 0.9848 | 0.9891 |
| Average boiling point, °C | 291.00 | 291.00 | 291.00 | 291.00 | 291.00 |
| BMCI | 65.2 | 75.3 | 76.3 | 95.9 | 98.0 |
Conclusions
Based on the results presented in the study, N,N-dimethylformamide (DMF) solvent appears to be the best solvent for selective solvent extraction among the five most widely used solvents in hydrocarbon industries. The degree of sulfur removal greatly depends on the nature of the solvent and operating conditions used during the extraction. Water amount in the solvent significantly changes the values of ESRGO yield and removal of impurities. Extraction temperature and water content in solvent give the flexibility to adjust the yield and the degree of removal of impurities to maximize the benefit under a given situation. Continuous counter current extraction is considerably more effective than the single stage extraction. 71.5% sulfur can be removed from SRGO using continuous counter current extraction at reasonable ESRGO yield. The selection of weight factor for sulfur removal and yield affects the performance factor of extraction process and needs the utmost care in its value selection. There is a great possibility of utilization of the extract as carbon black feed stock (CBFS) as shown by comparison of the calculated bureau of mines correlation index (BMCI) values which is similar to those CBFS which are already being sold in the market.References
- V. C. Srivastava, An evaluation of desulfurization technologies for sulfur removal from liquid fuels, RSC Adv., 2012, 2, 759–783 RSC.
- T. A. Koch, K. R. Krause, L. E. Manzer, M. Mehdizadeh, J. M. Odom and S. K. Sengupta, Environmental challenges facing the chemical-industry, New J. Chem., 1996, 20(2), 163–173 CAS.
- S. Kumar, V. C. Srivastava and R. P. Badoni, Oxidative desulfurization by chromium promoted sulfated zirconia, Fuel Process. Technol., 2012, 93, 18–25 CrossRef CAS PubMed.
- S. Kumar, V. C. Srivastava and R. P. Badoni, Oxidative desulfurization of dibenzothiophene by zirconia-based catalysts, Int. J. Chem. React. Eng., 2014, 12, 1–8 Search PubMed.
- M. Sharma, P. Sharma and J. N. Kim, Solvent extraction of aromatic components from petroleum derived fuels: a perspective review, RSC Adv., 2013, 3, 10103–10126 RSC.
- European emission standards, Wikipedia, http://en.wikipedia.org/wiki/European_emission_standards, accessed 22 March 2012.
- Clean fuel technology, http://www2.dupont.com/Clean_Technologies/en_US/assets/downloads/Hydrocarbon_Engineering_article_June_2009.pdf.
- R. Anantharaj and T. Banerjee, Aromatic sulfur-nitrogen extraction using ionic liquids: experiments and predictions using an a priori model, AIChE J., 2013, 59(12), 4806–4815 CrossRef CAS PubMed.
- J. Gao, H. Meng, Y. Lu, H. Zhang and C. Li, A carbonium pseudo ionic liquid with excellent extractive desulfurization performance., AIChE J., 2013, 59(3), 948–958 CrossRef CAS PubMed.
- Y. Shiraishi, K. Tachibana, T. Hirai and I. Komasawa, Desulfurization and denitrogenation process for light oils based on chemical oxidation followed by liquid-liquid extraction., Ind. Eng. Chem. Res., 2002, 41, 3662–4375 Search PubMed.
- P. Petkov, J. Tasheva and D. Stratiev, Extraction approach for desulphurization and dearomatization of middle distillates., Pet. Coal, 2004, 46, 13–18 CAS.
- A. A. Gaile, Development and improvement of extraction processes for separation and purification of petroleum products., Russ. J. Appl. Chem., 2008, 81, 1311–1324 CrossRef CAS.
- A. A. Gaile, V. E. Somov, G. D. Zalishchevskii, E. A. Kaifadzhyan and L. L. Koldobskaya, Extractive refining of atmospheric gas oil with N-methylpyrrolidone, Russ. J. Appl. Chem., 2006, 79, 590–595 CrossRef CAS.
- A. A. Gaile, B. M. Saifidinov, V. V. Kolesov and L. L. Koldobskaya, Extractive refining of high-sulfur diesel fraction to remove organic sulfur compounds and aromatic hydrocarbons, Russ. J. Appl. Chem., 2010, 83(3), 464–472 CrossRef CAS.
- A. A. Gaile, B. M. Saifidinov, V. V. Kolesov and L. L. Koldobskaya, Multistep countercurrent extraction of organic sulfur compounds and arenes from the high-sulfur diesel fraction, Russ. J. Appl. Chem., 2010, 83(3), 473–476 CrossRef CAS.
- V. Toteva, L. Topalova and P. Manolova, Extractive dearomatization and desulphurization of a distillate gasoil cut with imethylformamide, J. Univ. Chem. Technol. Metall., 2007, 42, 17–20 CAS.
- D. M. A. El-Aty, O. I. S. El.-Din, S. I. Hassa, S. M. Tawfik and S. Hanafi, Evaluation of some organic solvents for refining diesel fuel fraction, Pet. Sci. Technol., 2009, 27, 861–863 CrossRef.
- S. I. Hassan, O. I. S. El-Din and S. M. Tawfik, Solvent refining of straight run diesel fuel by various solvents phase equilibrium, J. Appl. Sci. Res., 2009, 5(5), 515–521 Search PubMed.
- B. S. Rawat, S. K. Ghosh and I. B. Gulati, Basic considerations in the selection of solvents for aromatics extraction, Pet. Hydrocarbons, 1972, 6, 203–210 CAS.
- B. S. Rawat and I. B. Gulati, Solvent for aromatics extraction and criteria for selection, J. Sci. Ind. Res., 1976, 35(6), 383 CAS.
- M. J. Hampe, Selection of solvents in liquid-liquid extraction according to physicochemical aspects, Chem. Eng. Technol., 1985, 57(8), 669–681 CAS.
- R. W. Cusack, P. Fremeaux and Y. N. V. Otto, A fresh look at liquid-liquid extraction, part 1: Extraction systems, Chem. Eng., 1991, 98(2), 66–76 CAS.
- R. W. Cusack and P. Fremeaux, A fresh look at liquid-liquid extraction, part 2: Inside the extractor, Chem. Eng., 1991, 98(3), 132–138 CAS.
- S. K. Ghosh, Solvents for petroleum industry, Hydrocarb Technol., 1993, 26, 13–19 Search PubMed.
- L. Alders, Liquid-Liquid Extraction–Theory and Laboratory Practice, Elsevier Publishing, 2nd edn, 1959 Search PubMed.
- Z. Ismagilov, S. Yashnik, M. Kerzhentsev, V. Parmon, A. Bourane, F. M. Al-Shahrani, A. A. Hajji and O. R. Koseoglu, Oxidative desulfurization of hydrocarbon fuels, Catal. Rev.: Sci. Eng., 2011, 53, 199–255 CAS.
- HPI Consultants Inc, Petroleum Refining Process correlations, 1996.
- Encyclopedia of Chemical. Processing and Design, ed. J. J. Mektta and W. A. Cunningham, Marcel Dekker, Inc., New York, 1990, vol. 6, p. 200 Search PubMed.
- CBFS specification, IOCL (Indian Oil Corporation Limited), http://www.iocl.com/Products/CarbonBlackFeedStockSpecifications.pdf, accessed 11 March 2014.
- CBFS specification, RIL (Reliance Industries Limited), http://www.ril.com/downloads/pdf/carbonstocksspecification.pdf, accessed 11 March 2014.
| This journal is © The Royal Society of Chemistry 2014 |