Selective fluorescence sensing of Mg2+ ions by Schiff base chemosensor: effect of diamine structural rigidity and solvent†
Abstract
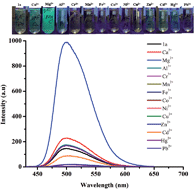
Highly selective strong turn-on fluorescence for Mg2+ (Φ = 0.03 to 0.57) was realized with a simple Salen based Schiff base chemosensor (1a) using dimethylformamide (DMF) or dimethyl sulfoxide (DMSO) as solvent. Importantly, Ca2+ that often interferes in the Mg2+ fluorescence sensing did not show any significant influence on the selectivity. The fluorescence sensing of Mg2+ is highly solvent as well as amine structure dependent. Fluorescence sensing of Mg2+ only by 1,2-phenylenediamine condensed Schiff bases in DMF or DMSO was observed. Different substituents (1b–e) on the salicylaldehyde unit were synthesized and we explored the effect of substitution on Mg2+ sensing and selectivity. Except 1c, other chemosensors showed selective fluorescence sensing of Mg2+ in DMF/DMSO. Interestingly, 1a–e (except 1c) exhibited selective strong turn-on fluorescence for Fe3+ (λmax = 462 nm, Φ = 0.421) in different solvents (DMSO, DMF, THF, CH3CN) after 1 h. The concentration dependent studies showed linear enhancement of fluorescence intensity for Mg2+ with the detection limit of 10−7 M. The practical applications of the chemosensor for selective sensing of Mg2+ in real samples such as pond, tap, river and ground water have also been demonstrated.

 Please wait while we load your content...
Please wait while we load your content...