Preparation of antifouling polyetherimide/hydrolysed PIAM blend nanofiltration membranes for salt rejection applications
Abstract
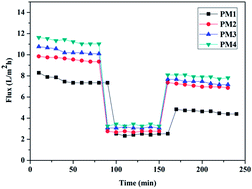
Nanofiltration (NF) membranes are continually sought for their unique physical and chemical properties, which allow filtration of electrolytes, dyes and other substances. In continuation of our efforts to prepare NF membranes, flat sheet polyetherimide/hydrolysed poly(isobutylene-alt-maleic anhydride) (PIAM) blend membranes have been prepared. The main aim was to explore the effect of addition of PIAM on morphological features and permeation properties of the membranes. The presence of dicarboxlic acid functionality leads to an enhancement in the hydrophilicity and antifouling properties. The results revealed that increasing the content of hydrolysed PIAM decreases the pore size of the membranes and subsequently increases the electrolyte rejection. The PEI/hydrolysed PIAM composition (80 : 20) showed reasonably good salt rejection (sodium sulphate of 1000 ppm) of up to 76% with a pure water flux of 11.8 L m−2 h−1 at 0.4 MPa transmembrane pressure. This study provides a simple and effective approach to produce a negatively charged NF membranes for water desalination applications with low energy consumption.

 Please wait while we load your content...
Please wait while we load your content...