Perspectives and advances of microalgal biodiesel production with supercritical fluid technology
Abstract
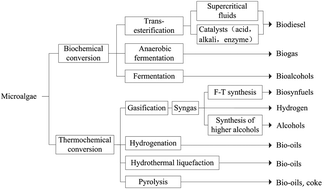
Biodiesel, a sustainable and clean energy source, has been greatly attracting interest to compete against serious challenges like energy crisis and environmental pollution. Microalgae are currently promoted as a biodiesel feedstock with the most potential and advantages of high lipid content and productivity. This paper provides an overview on a selection of microalgal strains, supercritical carbon dioxide (SCCO2) extraction of microalgal lipids and the advances of microalgae oils transesterification for producing biodiesel with supercritical alcohols. In particular, a two-step process of microalgal biodiesel production using supercritical technology and the following SCCO2 extraction are generalized in this study. Considering the commercialization of microalgal biodiesel in the future, the cost of microalgal biodiesel published in recent literature is analysed. Furthermore, feasible strategies for improvement are proposed. The overall economic efficiency of microalgal biodiesel industry can be improved by the multi-effect co-production coupling technology.

 Please wait while we load your content...
Please wait while we load your content...