Nanolayered manganese oxides as water-oxidizing catalysts: the effects of Cu(II) and Ni(II) ions†
Mohammad Mahdi Najafpour*ab,
Mahnaz Abasia and
Małgorzata Hołyńskac
aDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran. E-mail: mmnajafpour@iasbs.ac.ir; Tel: +98 241 415 3201
bCenter of Climate Change and Global Warming, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran
cFachbereich Chemie and Wissenschaftliches Zentrum für Materialwissenschaften (WZMW), Philipps-Universität Marburg, Hans-Meerwein-Straße, D-35032, Marburg, Germany
First published on 4th August 2014
Abstract
We synthesized nanolayered manganese oxides by the reaction of Mn(CH3COO)2·4H2O and KMnO4 in the presence of copper(II) or nickel(II) ions. The compounds were synthesized, and characterized by scanning electron microscopy, transmission electron microscopy, powder diffractometry and atomic absorption spectroscopy. Water-oxidizing activities of these oxides in the presence of cerium(IV) ammonium nitrate as a non-oxo transfer oxidant are reported. The results and proposed important factors influencing the water-oxidizing activities of these oxides are also discussed.
Introduction
H2 production on an industrial scale by water splitting is currently much discussed as a promising route for the conversion of sustainable but intermittent energy.1 However, the low rate of water oxidation and issues of long-term material stability are still not completely resolved.2 Different compounds were reported as efficient catalysts toward water oxidation but many of them are scarce and expensive.3 Among different compounds, Mn compounds are interesting4 because they are not only low-cost and environmentally friendly, but also used by Nature for biological water oxidation.5 Photosystem II has the unique capability to oxidize water to molecular oxygen in Nature.5 Photosystem II is a protein complex located in the thylakoid membrane of plants, algae, and cyanobacteria. In Photosystem II, enzymes capture photons of light to energize electrons that transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydronium and molecular oxygen. A Mn4O5Ca cluster housed in a special protein environment in Photosystem II, one calcium and four manganese ions, are bridged by five oxygen atoms. Four water molecules were also found in this structure6 that two of them are suggested as the substrates for water oxidation.In addition to Mn, Ca had been identified as an essential ion in the water-oxidizing complex (WOC) in Photosystem II, and Sr is the only cation, which can functionally substitute Ca in the WOC.7 A requirement for Ca for the functional assembly was proposed. Pecoraro and Brudvig's groups have also proposed the specific role for Ca in ligating one of the substrate water molecules.8 In the proposed mechanism, a ligated water or hydroxide ion forms the O–O bond of O2 by attacking the oxygen of a terminal Mn(V)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species in the final state of oxygen evolution reaction.8 Many Mn compounds were reported as structural models for the WOC in Photosystem II but a few of them are water-oxidizing catalysts toward water oxidation.4 Among these compounds, Mn oxides are good catalysts toward water oxidation.9 The layered Mn oxide with redox-inactive ions between their layers are promising for applications in water oxidation.9 In our previous papers, we show that generally redox-inert ions increase the water-oxidation activity of the Mn oxides, but the effect is not specific and many redox-inert ions show such effect.10 In this study, we try to answer this important question: do redox-active ions such as Cu(II) or Ni(II) have any special effect on the water-oxidizing activity of layered manganese oxides?
O species in the final state of oxygen evolution reaction.8 Many Mn compounds were reported as structural models for the WOC in Photosystem II but a few of them are water-oxidizing catalysts toward water oxidation.4 Among these compounds, Mn oxides are good catalysts toward water oxidation.9 The layered Mn oxide with redox-inactive ions between their layers are promising for applications in water oxidation.9 In our previous papers, we show that generally redox-inert ions increase the water-oxidation activity of the Mn oxides, but the effect is not specific and many redox-inert ions show such effect.10 In this study, we try to answer this important question: do redox-active ions such as Cu(II) or Ni(II) have any special effect on the water-oxidizing activity of layered manganese oxides?
Experimental
Materials
All reagents and solvents were purchased from commercial sources and were used without further purification.Synthesis of compounds
Solution 1: Cu(NO3)2·3H2O (see Table 1 for the amounts) or Ni(CH3COO)2·4H2O (see Table 1 for the amounts) and Mn(CH3COO)2·4H2O (1.41 mmol, 346 mg) were dissolved in the smallest amount of water.| Compound | Cu(NO3)2·3H2O (mg) for Mn–Cu oxide | Ni(CH3COO)2·4H2O (mg) for Mn–Ni oxide |
|---|---|---|
| 1-Cu/1-Ni | 27.7 (0.11 mmol) | 28.6 (0.11 mmol) |
| 2-Cu/2-Ni | 55.5 (0.23 mmol) | 57.3 (0.23 mmol) |
| 3-Cu/3-Ni | 111.0 (0.46 mmol) | 114.5 (0.46 mmol) |
| 4-Cu/4-Ni | 166.0 (0.69 mmol) | 171.6 (0.69 mmol) |
| 5-Cu/5-Ni | 277.0 (1.15 mmol) | 286.0 (1.15 mmol) |
| 6-Cu/6-Ni | 555.0 (2.3 mmol) | 572.0 (2.3 mmol) |
| 7-Cu/7-Ni | 1112.0 (4.61 mmol) | 1147.0 (4.61 mmol) |
Solution 2: to a solution of KMnO4 (1 mmol, 158 mg) in 30 mL water, KOH (16.83 g) was added to obtain a hot saturated KOH solution.
Addition of the solution 1 to the solution 2 under vigorous stirring resulted in a dark precipitate. After 1 h, the obtained suspension was filtered and washed using distilled water (1.0 L) before being allowed to dry for 12 h at 60 °C in an oven. The compounds were heated to higher temperatures (100–700 °C) for 10 h in air to obtain a brown powder.
Characterization
SEM was carried out with Philips CM120. For HRTEM and TEM, samples were placed on copper grids covered with carbon film and examined with a 300 keV Transmission electron microscope JEM-3010 UHR (Jeol Ltd., Japan), equipped with a retractable high-resolution slow scan CCD-Camera (Gatan Inc., USA) with GOS phosphorous scintillator and lanthanum hexaboride cathode as the electron source.The X-ray powder patterns were recorded with a Bruker, D8 ADVANCE (Germany) diffractometer (CuKα radiation). Atomic absorption spectroscopy (AAS) was performed on an Atomic Absorption Spectrometer Varian Spectr AA 110 to determine the content of manganese. Prior to each analysis, the analyzed oxide (2 mg) was added to concentrated nitric acid and H2O2, left at room temperature to ensure that the oxides were completely dissolved. The solutions were then diluted to 50 or 100 mL and analyzed by AAS.
Water oxidation
Oxygen evolution from aqueous solutions in the presence of cerium(IV) ammonium nitrate, (NH4)2Ce(NO3)6, (Ce(IV)) was measured using an HQ40d portable dissolved oxygen meter connected to an oxygen monitor with digital readout. The reactor was maintained at 25.0 °C in a water bath (Fig. S1†). In a typical run, the instrument readout was calibrated against air-saturated distilled water stirred continually with a magnetic stirrer in the air-tight reactor. After ensuring a constant baseline reading, the water in the reactor was replaced with Ce(IV) solution. Without catalyst, Ce(IV) was stable under this condition and oxygen evolution was not observed. After deaeration of the Ce(IV) solution with argon, Mn oxides as several small particles were added, and oxygen evolution was recorded with the oxygen meter under stirring. The formation of oxygen was followed, and oxygen formation rates per manganese ions (detected by AAS) were obtained from linear fits of the data.Results and discussion
We synthesized Mn oxides by the reaction of Mn(II) and MnO4−in concentrated KOH and in the presence of different amounts of Cu(II) or Ni(II). XRD data for the layered Mn oxide with redox-inactive ions show weak peaks at near 2θ ∼ 38°, observed in all octahedrally-coordinated Mn oxide materials,10 and the peak near 2θ ∼ 11° and 65°, found in most layered materials such as birnessite. It is interesting to note that when we synthesized Mn oxide in the presence of Cu(II) or Ni(II), other compounds were also formed (Fig. 1). Prepared 1-Cu–7-Cu at 60 °C are amorphous but layered Mn oxide is detectable (Fig. S4†). CuO is detectable in 7-Cu (Fig. S4†). Heating of the compounds at 200–300 °C causes a few CuMn2O4 formation that is detectable by XRD (Fig. S4†). At higher temperatures (>500 °C), layered Mn oxide converts to KMn8O16, and CuO is also formed. At 600 °C KMn8O16, CuO and CuMn2O4 are detectable (Fig. S4†). At 700 °C, Mn3O4 and CuMn2O4 are formed (Fig. S4†). 1-Ni–7-Ni prepared at 60 °C, similar to Cu, are amorphous. However, layered Mn oxide is detectable in all of them (Fig. S5†). Ni(OH)2 is detectable in 7-Ni (Fig. S5†). Heating of the compounds at 300 °C causes no fundamental changes for the layered Mn oxide (Fig. S5†). At higher temperatures, 500–600 °C, KMn8O16 and NiMnO3 are formed. At 700 °C, only NiMn2O4 is detectable (Fig. S5†).SEM images in all compounds show amorphous particles with diameter of less than 100 nm (Fig. 2). The samples of 4-Ni calcinated at 400 °C and 4-Cu at 200 °C comprise crystals of 30–250 nm diameters (Fig. 3a and b, S2 and S3†). Fig. 3c and d show TEM and HRTEM images of 4-Ni at 400 °C, where the crystal lattice planes visible at the edges correspond to the measured interplanar distances of ∼0.26 nm (corresponding to [104] in NiMnO3). It is confirmed by XRD.
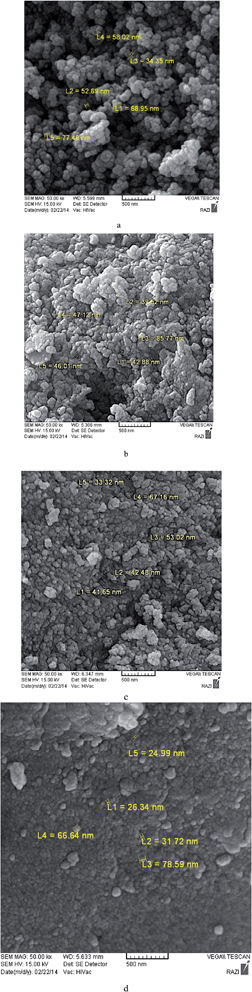 | ||
| Fig. 2 SEM images for calcinated 4-Cu at 200 °C (a), 7-Cu (b), calcinated 4-Ni at 400 °C (c) and 7-Ni (d). | ||
 | ||
| Fig. 3 TEM and HRTEM images of 4-Cu calcinated at 200 °C (a and b) and 4-Ni at 400 °C (c and d). (d) shows [104] in NiMnO3. | ||
The calcinated 4-Cu at 200 °C contains large crystals with round edges, apparently covered with a thin layer of amorphous substance (Fig. 3). The presence of amorphous part makes it difficult to follow the details of the crystalline part.
Water oxidation
(Ce(IV)) as a non-oxo transfer agent, soluble in water, stable and a strong one-electron oxidant is widely used as the primary oxidant in the oxidation of water to oxygen.9 As shown in Fig. 4, even low amounts of Cu(II) decrease water-oxidizing activity of the layered Mn oxide. However, Ni(II) in low amounts has less effect than Cu(II) on water oxidation. In both cases, a few increases in water oxidation were observed by increasing the concentration of Ce(IV).Temperature has no effect on water oxidation catalysed by 4-Ni. However, 4-Cu shows a higher activity at 200 °C. In consist with previously reported results,10 we found that the surfaces of compounds have low effect on water oxidation. For example, the surfaces of 4-Cu and 4-Ni are 118 and 26 m2 g−1 (Fig. S6†), respectively. However, water oxidation activities of these compounds are very similar (Fig. 4a).
On comparison with other layered Mn oxides (Table 2) (TOF ∼ 0.8–3), it is clear that Cu(II) or Ni(II) ions decreases water-oxidizing activity of the layered Mn oxides. Among different factors influencing water oxidation, it is also important to note that layered Mn oxides intercalated with different ions show different characteristics.
| Compound | Oxidant | TOF mmol O2/mol Mn | References |
|---|---|---|---|
| Optimistic Ca–Mn oxide | Ce(IV) | 3.0 | 12 |
| Nano scale Mn oxide within NaY zeolite | Ce(IV) | 2.62 | 13 |
| Layered Mn–Ca oxide | Ce(IV) | 2.2 | 14 |
| Layered Mn–Al, Zn, K, Cd and Mg oxide | Ce(IV) | 0.8–2.2 | 15 and 16 |
| Layered Mn–Cu(II) or Ni(II) oxide | Ce(IV) | 0.4–0.6 | This work |
| CaMn2O4·H2O | Ce(IV) | 0.54 | 17 |
| Amorphous Mn oxides | Ru(bpy)33+ | 0.06 | 18 |
| Ce(IV) | 0.52 | ||
| CaMn2O4·4H2O | Ce(IV) | 0.32 | 17 |
| Mn oxide nanoclusters | Ru(bpy)33+ | 0.28 | 19 |
| Mn oxide-coated montmorillonite | Ce(IV) | 0.22 | 20 |
| Layered Mn–Cu(II) or Ni(II) oxide | Ce(IV) | 0.2–0.35 | This work |
| Octahedral molecular sieves | Ru(bpy)33+ | 0.11 | 18 |
| Ce(IV) | 0.05 | ||
| MnO2 (colloid) | Ce(IV) | 0.09 | 21 |
| α-MnO2 nanowires | Ru(bpy)33+ | 0.059 | 22 |
| CaMn3O6 | Ce(IV) | 0.046 | 23 |
| CaMn4O8 | Ce(IV) | 0.035 | 24 |
| α-MnO2 nanotubes | Ru(bpy)33+ | 0.035 | 22 |
| Mn2O3 | Ce(IV) | 0.027 | 17 |
| β-MnO2 nanowires | Ru(bpy)33+ | 0.02 | 22 |
| Ca2Mn3O8 | Ce(IV) | 0.016 | 24 |
| CaMnO3 | Ce(IV) | 0.012 | 24 |
| Nano-sized λ-MnO2 | Ru(bpy)33+ | 0.03 | 25 |
| Bulk α-MnO2 | Ru(bpy)33+ | 0.01 | 22 |
| Mn complexes | Ce(IV) | 0.01–0.6 | 26 and 27 |
| PSII | Sunlight | 100–400 × 103 | 28 and 29 |
For example, Ca–Mn oxides are amorphous until ∼500 °C but similar compound containing K ions forms crystalline phase even at ∼300 °C.10,14–16 In contrast to the crystalline K–Mn oxide, anhydrous, amorphous and calcinated Ca–Mn oxide shows efficient water oxidation. Ni(II) ions in the presence of layered Mn oxides at 400–500 °C instead of modification of the layered structure forms crystalline NiMnO3 that is not an efficient water-oxidizing catalyst under our experimental conditions. Cu(II) has similar characteristics with formation of CuMn2O4 and CuO at 400–500 °C. Such phases are present even at lower temperatures. Under these conditions, some active Mn sites are consumed to form such inactive phases. Thus, we concluded that where the layered structures are stable at 300–500 °C, we expect high water oxidation rates by Mn oxides.10 The active catalysts have layered structure but the layers did not display any long-range order. Using TEM and electron scattering simulations, Chang and Spiccia indicated that the birnessite phase with high degree of layer mis-registration, and a high concentration of Mn vacancies are efficient catalysts toward water oxidation.11 We also proposed that the activities may correlate with the degree of disorder in the layered Mn oxide. On the other hand, covering active sites on the surface of layered Mn oxide by Cu(II) or Ni(II) ions can decrease water oxidation activity. In the case of Cu(II) or Ni(II) ions, perturbation in water oxidation by these redox-active ions is also possible.
Conclusions
Layered manganese oxides were synthesized by the reaction of Mn(CH3COO)2·4H2O and KMnO4 in the presence of copper(II) or nickel(II) ions. The compounds were characterized by SEM, XRD, AAS and TEM. These compounds were tested as catalysts of water oxidation in the presence of Ce(IV). In comparison with other layered Mn oxides, we found that Cu(II) or Ni(II) decrease the water-oxidizing activity of the layered Mn oxides. We suggest that Ni(II) or Cu(II) ions in the presence of layered Mn oxides instead of modification of the layered structure for water oxidation form other phases that are not efficient as water-oxidizing catalysts. The results could show that redox-active cations may decrease water-oxidizing activity of these oxides.Acknowledgements
MMN and MA are grateful to the Institute for Advanced Studies in Basic Sciences and the National Elite Foundation for financial support. MH acknowledges Prof. Dr S. Dehnen for generous support and helpful discussions.Notes and references
- J. A. Turner, Science, 1999, 285, 687 CrossRef CAS; T. J. Meyer, Nature, 2008, 451, 778 CrossRef PubMed; T. R. Cook, D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets and D. G. Nocera, Chem. Rev., 2010, 110, 6474 CrossRef PubMed; N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729 CrossRef PubMed; T. Faunce, S. Styring, M. R. Wasielewski, G. W. Brudvig, A. W. Rutherford, J. Messinger, A. F. Lee, C. L. Hill, H. deGroot, M. Fontecave, D. R. MacFarlane, B. Hankamer, D. G. Nocera, D. M. Tiede, H. Dau, W. Hillier, L. Wang and R. Amal, Energy Environ. Sci., 2013, 6, 1074 Search PubMed.
- D. G. Nocera, Acc. Chem. Res., 2012, 45, 767 CrossRef CAS PubMed.
- Critical raw materials for the E, Report of the Ad-hoc Working Group on defining critical raw materials, European Commission 2010; Studie des IZT und ISI im Auftrag des BMWi, Schlussbericht: Rohstoffe für Zukunftstechnologien 2009.
- A. Singh and L. Spiccia, Coord. Chem. Rev., 2013, 257, 2607 CrossRef CAS PubMed; W. Ruttinger and G. C. Dismukes, Chem. Rev., 1997, 97, 1 CrossRef PubMed; M. Yagi and M. Kaneko, Chem. Rev., 2001, 101, 21 CrossRef PubMed; H. J. M. Hou, J. Integr. Plant Biol., 2010, 52, 704 CrossRef PubMed; H. J. M. Hou, Materials, 2011, 4, 1693 CrossRef PubMed; K. Beckmann, H. Uchtenhagen, G. Berggren, M. F. Anderlund, A. Thapper, J. Messinger, S. Styring and P. Kurz, Energy Environ. Sci., 2008, 1, 668 Search PubMed; M. Hirahara, A. Shoji and M. Yagi, Eur. J. Inorg. Chem., 2014, 4, 595 CrossRef PubMed.
- V. K. Yachandra, V. J. DeRose, M. J. Latimer, I. Mukerji, K. Sauer and M. P. Klein, Science, 1993, 260, 675 Search PubMed; J. E. Penner-Hahn, Structural characterization of the Mn site in the photosynthetic oxygen-evolving complex, in Metal Sites in Proteins and Models: Redox Centres, ed. H. A. O. Hill, P. J. Sadler and A. J. Thomson, Springer, Berlin, Heidelberg, Germany, 1998, vol. 90, pp. 1–36 CrossRef CAS; H. Dau, L. Iuzzolino and J. Dittmer, Biochim. Biophys. Acta, 2001, 1503, 24 CrossRef CAS; K. Sauer, J. Yano and V. K. Yachandra, Coord. Chem. Rev., 2008, 252, 318 CrossRef PubMed; A. Zouni, H. T. Witt, J. Kern, P. Fromme, N. Krauss, W. Saenger and P. Orth, Nature, 2001, 409, 739 CrossRef PubMed; N. Kamiya and J.-R. Shen, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 98 CrossRef PubMed; K. N. Ferreira, T. M. Iverson, K. Maghlaoui, J. Barber and S. Iwata, Science, 2004, 303, 1831 CrossRef PubMed; B. Loll, J. Kern, W. Saenger, A. Zouni and J. Biesiadka, Nature, 2005, 438, 1040 CrossRef PubMed; J. Yano, J. Kern, K. Sauer, M. J. Latimer, Y. Pushkar, J. Biesiadka, B. Loll, W. Saenger, J. Messinger and A. Zouni, Science, 2006, 314, 821 CrossRef PubMed; J. W. Murray, K. Maghlaoui, J. Kargul, N. Ishida, T.-L. Lai, A. W. Rutherford, M. Sugiura, A. Boussac and J. Barber, Energy Environ. Sci., 2008, 1, 161 Search PubMed; K. Kawakami, Y. Umena, N. Kamiya and J. R. Shen, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 8567 CrossRef PubMed; A. Guskov, J. Kern, A. Gabdulkhakov, M. Broser, A. Zouni and W. Saenger, Nat. Struct. Mol. Biol., 2009, 16, 334 Search PubMed; M. Broser, C. Glöckner, A. Gabdulkhakov, A. Guskov, J. Buchta, J. Kern, F. Müh, H. Dau, W. Saenger and A. Zouni, J. Biol. Chem., 2011, 286, 15964 CrossRef PubMed; S. I. Allakhverdiev, Photosynth. Res., 2008, 98, 1 CrossRef PubMed; S. I. Allakhverdiev, J. Photochem. Photobiol., B, 2011, 104, 1 CrossRef PubMed; E. M. Sproviero, J. A. Gascón, J. P. McEvoy, G. W. Brudvig and V. S. Batista, J. Am. Chem. Soc., 2008, 130, 3428 CrossRef PubMed; J. Yano and V. Yachandra, Chem. Rev., 2014, 114, 4175 CrossRef PubMed.
- Y. Umena, K. Kawakami, J. R. Shen and N. Kamiya, Nature, 2011, 473, 55 CrossRef CAS PubMed; Y. Umena, N. Kamiya and J. R. Shen, J. Photochem. Photobiol., B, 2011, 104, 9 CrossRef PubMed.
- J. P. McEvoy and G. W. Brudvig, Chem. Rev., 2006, 106, 4455 CrossRef CAS PubMed.
- V. L. Pecoraro, M. J. Baldwin, M. T. Caudle, W. Y. Hsieh and N. A. Law, Pure Appl. Chem., 1998, 70, 925 CrossRef CAS; J. Limburg, V. A. Szalai and G. W. Brudvig, J. Chem. Soc., Dalton Trans., 1999, 1353 RSC.
- T. S. Glikman and I. S. Shcheglova, Kinet. Katal., 1968, 9, 461 Search PubMed; M. Morita, C. Iwakura and H. Tamura, Electrochim. Acta, 1977, 22, 325 CrossRef CAS; A. Harriman, I. J. Pickering, J. M. Thomas and P. A. Christensen, J. Chem. Soc., Faraday Trans. 1, 1988, 84, 2795 RSC; K. L. Pickrahn, S. W. Park, Y. Gorlin, H. B. R. Lee, T. F. Jaramillo and S. F. Bent, Adv. Energy Mater., 2012, 2, 1269 CrossRef PubMed; D. M. Robinson, Y. B. Go, M. Mui, G. Gardner, Z. Zhang, D. Mastrogiovanni, E. Garfunkel, J. Li, M. Greenblatt and G. C. Dismukes, J. Am. Chem. Soc., 2013, 135, 3494 CrossRef PubMed; M. M. Najafpour, A. N. Moghaddam, H. Dau and I. Zaharieva, J. Am. Chem. Soc., 2014, 136, 7245 CrossRef PubMed; M. Wiechen, M. M. Najafpour, S. I. Allakhverdiev and L. Spiccia, Energy Environ. Sci., 2014, 7, 2203 Search PubMed.
- M. Najafpour, S. Heidari, E. Amini, M. Khatamian, R. Carpentier and S. I. Allakhverdieve, J. Photochem. Photobiol., B, 2014, 133, 124 CrossRef CAS PubMed.
- S. L. Y. Chang, A. Singh, R. K. Hocking, C. Dwyer and L. Spiccia, J. Mater. Chem. A, 2014, 2, 3730 CAS.
- C. E. Frey, M. Wiechen and P. Kurz, Dalton Trans., 2014, 4370 RSC.
- M. M. Najafpour and B. Pashaei, Dalton Trans., 2012, 10156 RSC.
- M. M. Najafpour, S. Nayeri and B. Pashaei, Dalton Trans., 2011, 9374 RSC.
- M. M. Najafpour, B. Pashaei and S. Nayeri, Dalton Trans., 2012, 7134 RSC.
- M. M. Najafpour, D. Jafarian Sedigh, B. Pashaeia and S. Nayeri, New J. Chem., 2013, 37, 2448 RSC.
- M. M. Najafpour, T. Ehrenberg, M. Wiechen and P. Kurz, Angew. Chem., Int. Ed., 2010, 49, 2233 CrossRef CAS PubMed.
- A. Iyer, J. Del-Pilar, C. Kithongo King'ondu, E. Kissel, H. Fabian Garces, H. Huang, A. M. El-Sawy, P. K. Dutta and S. L. Suib, J. Phys. Chem. C, 2012, 116, 6474 CAS.
- Y. Okuno, O. Yonemitsu and Y. Chiba, Chem. Lett., 1983, 815 CrossRef CAS.
- M. M. Najafpour and A. N. Moghaddam, New J. Chem., 2012, 36, 2514 RSC.
- M. M. Najafpour, Dalton Trans., 2011, 3805 RSC.
- V. B. R. Boppana and F. Jiao, Chem. Commun., 2011, 47, 8973 RSC.
- M. M. Najafpour, Dalton Trans., 2011, 3793 RSC.
- M. M. Najafpour, S. Nayeri and B. Pashaei, Dalton Trans., 2012, 4799 RSC.
- D. M. Robinson, Y. B. Go, M. Greenblatt and G. C. Dismukes, J. Am. Chem. Soc., 2010, 132, 11467 CrossRef CAS PubMed.
- C. W. Cady, R. H. Crabtree and G. W. Brudvig, Coord. Chem. Rev., 2008, 252, 444 CrossRef CAS PubMed.
- R. Ramaraj, A. Kira and M. Kaneko, Angew. Chem., 1986, 98, 824 CrossRef CAS PubMed.
- G. C. Dismukes, R. Brimblecombe, G. A. N. Felton, R. S. Pryadun, J. E. Sheats, L. Spiccia and G. F. Swiegers, Acc. Chem. Res., 2009, 42, 1935 CrossRef CAS PubMed.
- G. Ananyev and G. C. Dismukes, Photosynth. Res., 2005, 84, 355 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05617e |
| This journal is © The Royal Society of Chemistry 2014 |


