Gel, glass and nematic states of plate-like particle suspensions: charge anisotropy and size effects
Abstract
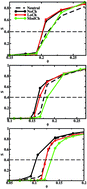
The influence of the charge anisotropy and platelet size on the formation of gel and glass states and nematic phases in suspensions of plate-like particles is investigated using Monte Carlo simulations in the canonical ensemble. The platelets are modeled as discs with charged sites distributed on a hexagonal lattice. The edge sites can carry a positive charge, while the remaining sites are negatively charged giving rise to a charge anisotropy. A screened Coulomb potential plus a short range repulsive potential are used to describe the interactions between the sites of the platelets. The liquid–gel transition is found to be favored by a high charge anisotropy and by large particles. Oppositely, the liquid–glass transition is favored for small particles without charge anisotropy, i.e., fully negatively charged. Finally, we find that the isotropic/nematic transition is disfavored by the charge anisotropy. For a strong charge anisotropy, the nematic phase does not form and, instead, a gel/columnar transition is found.

 Please wait while we load your content...
Please wait while we load your content...