Fabrication temperature modulates bulk properties of polymeric gels synthesized by different crosslinking methods
Deepak Bushan Raina†
,
Raman Koul†,
Aniket Bangroo and
Ashok Kumar*
Department of Biological Sciences and Bioengineering, DST Unit of Nanoscience and Center for Environmental Science and Engineering, Indian Institute of Technology Kanpur, Kanpur 208016, India. E-mail: ashokkum@iitk.ac.in; Tel: +915122594051
First published on 2nd July 2014
Abstract
The effects of a natural matrix like extracellular matrix (ECM) on cell growth, proliferation and induction of mechanotransduction signals are well documented. Mimicking the mechanical and rheological character of ECM to repair, reconstruct and regenerate injured or impaired tissue using the concepts of tissue engineering is of significant importance. We intended to study the effect of “synthesis temperature” on mechanical and rheological properties of monomeric and polymeric scaffolds fabricated from either monomer based polyacrylamide gels or by physical gelation (agarose gels) or chemical crosslinking (chitosan, chitosan–gelatin and chitosan–agarose–gelatin (CAG) gels) at −15 °C, −5 °C or 25 °C. Our results indicated that in the absence of porogens in the form of ice crystals, conventional hydrogels had higher mechanical strength and low porosity as compared to cryogels synthesized at −15 °C and −5 °C. Furthermore, rheological analysis performed on these gels indicated temperature dependence of rheology. CAG cryogels had higher storage modulus as compared to freeze dried (FD) hydrogels or conventional hydrogels in the wet state making them amiable for cartilage tissue regeneration. Out of all synthesized gels, CAG showed better mechanical and rheological properties and was further studied to analyze the interaction with primary chicken chondrocytes. In vitro experiments on CAG scaffolds revealed a significant amount of ECM production along with good cell proliferation as indicated by MTT assay and SEM analysis.
1. Introduction
The main objective of tissue engineering is to regenerate, reconstruct, and/or replace damaged tissue/organ by mimicking native tissue as closely as possible. To accomplish these goals it is imperative to combine cells within natural or synthetic scaffolds that help cells to exist in their native environment. Cells secrete various molecules that make a variety of natural scaffolds. These scaffolds govern the attachment, organization, and proliferation of cells to fabricate biologically functional tissue(s). Extracellular matrix (ECM) acts as a scaffold since it is a natural, three dimensional (3D) environment that provide cells an attachment platform in order to maintain tissue's structural integrity and its biological functions. ECM also acts as an instruction manual for cells guiding them during different chemical, electrochemical and physical stimuli to control cellular growth, differentiation and migration, thus exhibiting mechanotransductive behaviour.1–3Cell–ECM interactions are of paramount importance and have been the research focus of various scientific communities across different fields including material science, regenerative medicine and tissue engineering. Researchers for long have tried to harness and mimic these interactions using various natural and synthetic scaffolds.4,5 A key component of tissue engineering is the fabrication of 3D scaffold that not only provide a 3D environment to support cellular growth and proliferation, but also mimics many other functions of ECM including differential and dynamic mechanical behaviour based on tissue type.6
In vivo differential and dynamic mechanical property of ECM across tissues at different stages of life has shown to influence cell proliferation, migration, and differentiation.7–9 Furthermore, the role of mechnotransduction in stem cell lineage specification, cell migration and disease in tissues or organs like bone, cartilage, skin, muscle, brain, eye, etc. is also well documented.3,10–14 Recently, independent of its chemical nature, the mechanical properties of ECM have been recognized as vital in regulating cellular functions through physical couplings that associate the ECM to the cell cytoskeleton. These characteristics allow for direct translation of force into remodelling of cellular gene expression and phenotype.15–17 Conversely, the mechanical properties of ECM can thus be modulated by a variety of covalent and physical interactions between the individual ECM constituent molecules. One of the frequently used parameter for the measurement for mechanical strength or rigidity of tissues is the elastic (Young's) modulus. Elastic modulus of various tissues like brain, lungs, breast, muscle, fat, and bone lie in the range of 0.1 kPa to 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kPa. Recent studies have used various tissue engineering scaffolds including hydrogels, Matrigel™, crosslinked gelatin, and other synthetic substrates with elastic moduli in the same range that of native tissue to mimic native environment and thus influence cellular outcomes.18 Thus, a major challenge for researchers is to incorporate identical or at least similar mechanical properties to their scaffolds just like the native tissue.19
000 kPa. Recent studies have used various tissue engineering scaffolds including hydrogels, Matrigel™, crosslinked gelatin, and other synthetic substrates with elastic moduli in the same range that of native tissue to mimic native environment and thus influence cellular outcomes.18 Thus, a major challenge for researchers is to incorporate identical or at least similar mechanical properties to their scaffolds just like the native tissue.19
Considerable advances in designing of artificial matrices, those can mimic properties of ECM paved a way for evolution of much more dynamic scaffolds. An enhanced attention is paid for the matrices those not only support cell growth and proliferation, but also exhibit efficient nutrient transfer, gas exchange (i.e., O2 and CO2) and metabolic waste removal together with efficient signal transduction. Scaffolds in various forms have been synthesized, studied and employed for different tissue regeneration purposes.20 Hydrogels have been one of the earliest and well studied forms of biocompatible tissue engineering gels that have proven their worth to mimic ECM like properties.21 Hydrogels have excellent water uptake and retention property, which allows free diffusion of solute molecules. Various forms of hydrogels have thus far been synthesized from various sources (natural/synthetic polymers) or/and with varied crosslinking methods (monomer based, chemically cross linked and physically cross linked) for tissue engineering or biomedical use.22 The use of free-radical cross-linking copolymerization of a hydrophilic, non-ionic monomer has been used as the most common way to produce these gels. Thus, the hydrogel's structure and its property including its mechanical strength are function of concentration of crosslinker, chemical nature and initial concentration of the monomer units. However, despite of its wide application range, its use has been limited in medical and biological field by a number of limitations including inhomogeneity and poor/imbalanced mechanical strength.23–25 Thus, designing of polymeric gels with a balanced mechanical strength is vital for certain existing as well as developing applications. One of the techniques to obtain gels with fast response time and good mechanical strength is cryogelation.26 This technique produces highly interconnected, macroporous structures due to phase separation at cryo-conditions. The phenomenon of phase separation imparts cryogels with fast responsive time and a good mechanical strength as compared to macroporous hydrogels.27–30 Cryogels thus far have been used in varied biological applications like cell separation, neo-cartilage formation, cell proliferation and stimulation.31–34
In the present work, we have performed an in-depth analysis of rheological and mechanical properties of monomer based, chemically crosslinked, and physically crosslinked, porous scaffolds fabricated at −15 °C, −5 °C and 25 °C. Furthermore, we also evaluated proliferative differences of chicken derived chondrocytes on chitosan, chitosan–gelatin, and chitosan–agarose–gelatin cryogels by varying polymeric component thus influencing mechanical strength, elasticity and porosity.
2. Materials and methods
2.1 Materials
Acrylamide, ammonium persulfate (APS) and glutaraldehyde (25%) were purchased from MERCK India Pvt. Ltd., Mumbai, India. N,N′-Methylenebisacrylamide and tetramethylethylene-diamine (TEMED) were purchased from SD fine chemicals, India. Gelatin, from coldwater fish, low viscosity chitosan, Dulbecco's modified eagle's medium (DMEM) high glucose, [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT), trypsin solution, and collagenase type II were purchased from Sigma Aldrich, U.S.A. Fetal bovine serum (FBS) from Gibco Life Technologies, U.S.A, while low eeo agarose was purchased from SRL labs, India.2.2 Methods
2.2.2.1 Preparation of chitosan cryogels and hydrogels. Chitosan cryogels were prepared by dissolving 200 mg (2% w/v) of low viscosity chitosan in 8 ml of 1% acetic acid. The polymeric solution was left overnight on rocker to ensure complete dissolution of the polymer. The solution was then precooled to 4 °C for 30 min. Meanwhile, 0.2% (v/v) of the crosslinker glutaraldehyde (25%) was made in 2 ml of 1% acetic acid and added to the chilled polymeric solution to make up the final volume to 10 ml. This viscous polymeric solution was homogenized by vortex mixing for 15 s. The solution was then poured into precooled 2.5 ml syringe moulds and incubated in the circulating liquid cryostat maintained at either −5 °C or −15 °C for 12–16 h. The gels were then thawed in de-ionized water for 2–3 h and dried in a similar manner as specified above. Chitosan FD hydrogels (2%) and conventional chitosan hydrogels (4%) were fabricated by following the same protocol as mentioned in Section 2.2.1. The concentration of conventional hydrogels has been optimized in order to ensure better handling during different analysis techniques.
2.2.2.2 Preparation of chitosan–gelatin cryogels and hydrogels. Chitosan 200 mg (2% w/v) was dissolved in 9 ml of 1% acetic acid followed by the addition of 600 mg (6% w/v) gelatin. The polymers were left overnight on rocker to homogenize completely. The polymer solution was then precooled at 4 °C. Glutaraldehyde 0.2% (v/v) was mixed with 1 ml of 1% acetic acid and added to the polymeric solution to make up the total reaction mixture volume to 10 ml. The solution was then vortexed thoroughly followed by pouring into precooled 2.5 ml syringe moulds. The moulds were incubated in liquid circulating cryostat maintained either at −5 °C or −15 °C for 12–16 h. Gels were thawed and dried as mentioned earlier. Chitosan–gelatin hydrogels (both conventional and FD) were fabricated at 25 °C by following above-mentioned protocol and by maintaining same polymeric concentration as above. Air-dried hydrogels were dried at room temperature while FD hydrogels were dried using lyophilization.
2.2.2.3 Preparation of chitosan–agarose–gelatin cryogels and hydrogels. Chitosan–agarose–gelatin (CAG) cryogels having 13% (w/v) of total gel strength were prepared by dissolving 200 mg (2% w/v) chitosan in 5 ml of 1% acetic acid. Upon complete dissolution of chitosan, gelatin 600 mg (6% w/v) was added to the chitosan solution and kept on rocker for complete dissolution. Additionally, agarose 500 mg (5%) was dissolved in 4 ml of de-ionized water by heating it to 80 °C for half an hour. After complete dissolution, chitosan–gelatin solution was added to the agarose solution and the mixture was allowed to homogenize by heating at 60 °C for 30 min. Once all the polymers were mixed thoroughly, the mixture was allowed to cool to 40 °C followed by the addition of 0.2% (v/v) glutaraldehyde (25%). The resultant mixture was vortexed vigorously for 10 s and transferred into plastic moulds. The moulds were immediately transferred into a liquid cryostat maintained at either −5 °C or −15 °C. The drying procedure was not changed and freeze-drying was used to dry the cryogels. CAG hydrogels were also synthesized exactly the same way with variation only in the temperature. The reaction was allowed to occur at room temperature (25 °C), rather than at sub-zero temperatures. However, the hydrogels were dried either using freeze-drying or air-drying methods as mentioned earlier.
2.3 Characterization of synthesized cryogels and hydrogels
The % water retention capacity of the gels was calculated using the following formula:
| WR (%) = (Wtp − Wdry)/Wwet × 100 |
| Average pore volume = (Wwet − Wdry)/Wwet × 100 |
2.4 Cell–material interaction
The assay was performed in triplicates to reduce the error. On the day of the assay, culture media was removed from each well, followed by the addition of MTT reagent (0.5 mg ml−1 in plain DMEM). MTT treated samples were then incubated for 4–5 h at 37 °C. The MTT reagent was aspirated from each well followed by the addition of 1.5 ml dimethylsulfoxide (DMSO) per well. The plates with DMSO were incubated for 15–20 min. After incubation, DMSO was carefully pipetted out into 2 ml micro-centrifuge tubes and absorbance was read at 570 nm against DMSO as a blank. Absorbance vs. time histogram was plotted for both control and test samples in order to access the cell proliferation and biocompatibility of the synthesized matrices.
3. Results and discussions
In our current work we have primarily focused on two different types of gels namely, cryogels and hydrogels. Cryogelation is a relatively new technique in which the polymeric solution is incubated under sub-zero temperatures and rapid phase separation takes place which leads to the formation of ice crystals in frozen liquid phase. During this process polymer concentrates in the unfrozen liquid microphase by the phenomenon of cryoconcentration. Presence of a crosslinker in the mixture then leads to crosslinking of the polymer, which results in the formation of a highly interconnected porous network. The ice crystals upon thawing give rise to a porous and interconnected 3D macroporous structure known as cryogels. On the contrary, in case of hydrogels, the polymeric solution and the crosslinker react at room temperature to form a polymeric network, which is comparatively brittle, less porous and poorly interconnected due to lack of phase separation process. We have characterized these gels by different techniques that are discussed in detail in the following sections. In the latter part of this work, we choose to evaluate the potential of natural polymer based cryogel scaffold in supporting cell growth. However, these scaffolds varied from each other in terms of polymeric components, while all other parameters remained unchanged, which led to varied rheological and mechanical behaviour of the materials due to a change in the precursors. We primarily focused on chitosan, chitosan–gelatin and CAG cryogel scaffolds for analysing the proliferation of chicken chondrocytes. CAG scaffolds have already been known to support the proliferation of chondrocytes and aid in the development of neo-cartilage.34 However, our aim was to compare the effect of addition of polymeric components to basic chitosan cryogels to achieve effective cell proliferation capability of CAG cryogel scaffolds. Chitosan and gelatin mimic the protein component of the ECM while agarose adds to the mechanical strength and elasticity of the scaffolds.32 The motivation of doing this study was to investigate the importance of mechanical and rheological properties of a 3D scaffold on cell proliferation. In the cell–material interaction section, these properties have been tailored by majorly varying the temperature and polymeric components of cryogels thus emphasizing the novelty of this research work.3.1 Synthesis of cryogels and hydrogels
Fig. 1 represents digital images of some of the polymeric gels described earlier at different temperatures and by using different crosslinking methods. The monomer based, 6% pAAM gels were synthesized by free radical polymerization.33 While as, three different types of chemically crosslinked gels i.e. 2% and 4% chitosan, 8% chitosan–gelatin and 13% CAG gels were synthesized utilizing Schiff base reaction. Schiff base reaction crosslinks aldehyde groups of glutaraldehyde and free amino groups of chitosan and gelatin. As agarose (low EEO) exhibits self gelation property below 40 °C, 5% agarose gels were synthesized without the use of a crosslinker. All the gels were synthesized by varying the temperature. Three temperatures, −15 °C, −5 °C and 25 °C were used to observe the effect of temperature on the bulk properties of cryogels. Chitosan hydrogels have been synthesized at two different concentrations owing to their structural stability at higher concentration thus making handling easier. We used 2% FD chitosan hydrogels and 4% conventional chitosan hydrogels. While cryogelation imparts well-defined pore morphology, high interconnectivity, high elasticity and mechanical stability owing to cryo-structuring, we focused on studying the difference in bulk properties of cryogels and hydrogels synthesized with same monomer/polymer concentration. However, the only difference that makes these fabrication techniques contrasting is the variation in the synthesis temperature, which is one of the motivations behind this work.3.2 Scanning electron microscopy (SEM)
In order to study the effect of temperature on the pore morphology of the gels, SEM analysis was performed (Fig. 2). A common trend was observed among all gel types fabricated at different temperatures. The gels synthesized at −15 °C had open and homogenously distributed pore morphology (Fig. 2A) as compared to the gels synthesized at −5 °C and 25 °C (Fig. 2B–D). Moreover, the pore size of the gels made at −15 °C was bigger than the gels synthesized at other temperature which ensures convective fluid flow through cryogel monoliths. This can be attributed to the fact that the phase separation i.e. the formation of ice crystals and unfrozen liquid microphase takes place very fast. The ice crystals are formed rapidly in the frozen phase while polymerization and/or crosslinking take place in unfrozen liquid microphase. After phase separation, the solute molecules begin migrating leading to the formation of a cryo-concentrate.Moreover, under sub-zero temperatures, due to the cryo-concentration the rate of reaction also accelerates allowing the monomers/polymers to react rapidly. Meanwhile, the ice crystals keep growing from the periphery of the mould towards the center till they meet the facet of another crystal.28 This phenomenon of cryo-structuring ensures large pore size in case of cryogels. Additionally, the pore size in case of cryogels also depends on a number of factors like, concentration of the precursors, amount of crosslinker, freezing temperature, etc. thus cryogels can be tailored as per the desired application. Even though, the SEM analysis reveals that the pore morphology of cryogels synthesized at −15 °C is more prominent and well defined, it should be noted that the size of ice crystals formed at −5 °C is generally larger than the crystals formed at −15 °C and the pore size is bigger at higher freezing temperatures when compared with low freezing temperature.35 However, the crystals are not able to branch or propagate in an efficient manner due to uncontrolled nucleation and also due to depression in freezing point leading to rough and closed morphology of pores. However, in our experiments, gels synthesized are more porous and well defined at −15 °C because of the significant depression in the freezing point that happens at −5 °C. This freezing point depression results in the formation of a new phase known as the vitreous or glass phase, which leads to the formation of smaller pores.
In case of conventional hydrogels, lack of ice crystals leads to the absence of a physical barrier between polymeric chains, causing them to come closer thus resulting in the formation of significantly smaller pores (Fig. 2D). However, a comparison between the pore morphology of conventional and FD hydrogels indicates that FD gels possess some pores (Fig. 2C). This difference in pore morphology is caused by the freezing of the free water in FD gels, which leads to formation of pores upon lyophilization. The transport of solute molecules in conventional hydrogels mainly takes place because of the space that exists between the polymeric chains and there exist a number of theories that govern the movement of molecules.36
Thus, it can be concluded from the SEM results that the cryogels synthesized at −15 °C has larger, well-defined and interconnected pores because of proper crystallization under cryo-conditions throughout the gel. The gels synthesized at −5 °C and 25 °C have smaller pores due to the depression in freezing point and lack of ice crystal formation, respectively. The conventional hydrogels, being dried in a vacuum desiccator rather than lyophilizing leads to the formation of very small and less interconnected pores as observed in the SEM analysis.
3.3 Rheological analysis
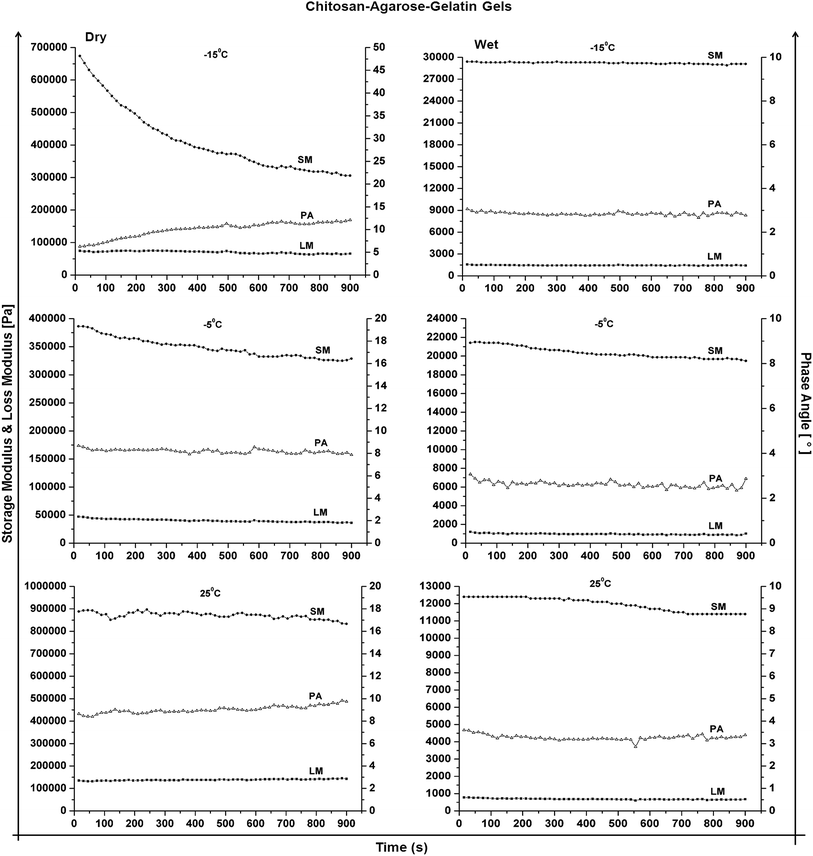
Rheology can be defined as the measure of viscoelastic properties of a material or the study that analyses the flow or deformation of materials when there is an impending stress. The main objective of studying rheology in this work is to explain how various parameters like temperature, type of monomeric/polymeric precursors, source of the precursors i.e. natural or synthetic, state of the material i.e. dry or wet affect the rheological properties of a material. The storage modulus (G′) of a material is basically the measure of the energy that a material can store upon application of certain force. This energy is however, transformed into elasticity of the material. The loss modulus (G′′) of a material is the viscous behaviour of the material when a force is applied. The force applied causes the material to flow and thus there is loss of energy while the material flows. This energy lost is quantified by the loss modulus. The phase angle (δ) is also another important parameter defining the rheological properties of the material. The phase angle is basically the difference in waveform shift that is recorded when a force is applied and a response to the force is generated. A phase angle value closer to 0° indicates that a material is more elastic while as the phase angle value closer to 90° indicates viscous nature of a material. In our study, we have compared different types of gels synthesized at different temperatures to observe the effect of different crucial parameters like temperature, concentration, etc. on the rheological properties of polymeric gels.Since the water uptake of both the gels was different, it might be possible that this unequal uptake of water balances their elastic properties in the wet state, which yields nearly similar storage modulus in these gels. In case of CAG cryogels (Fig. 6), the effect of adding agarose is clearly visible. Unlike chitosan and chitosan–gelatin gels, we observed a difference in the storage moduli of both cryogels and FD hydrogel. The storage modulus is least in case of CAG FD hydrogels due to a very high concentration. CAG cryogels made at −15 °C are the most elastic of the lot in the wet state. This is one of the most important reasons of choosing CAG cryogels for regenerating the elastic cartilage tissue and also studying the effect of ECM production on viscoelastic and mechanical properties of CAG cryogels. All chemically crosslinked conventional hydrogels (Fig. 4) in the wet state exhibit an increasing trend of storage modulus with addition of another polymer with CAG hydrogel with maximum storage modulus values and chitosan the least.
3.4 Mechanical analysis of cryogels and hydrogels
Fig. 9–11 indicate the mechanical behaviour of different polymeric gels made at varying temperatures. The mechanical properties of the gels were tested on a uniaxial Bose Electroforce mechanical analyser. The ratio of height and diameter was kept same in all the gels. The gels were placed on the load cell of the machine while uniaxial force was applied from the top. The ramp moved at a speed of 0.5 mm s−1. The output generated from the tests was then reconverted in terms of stress and strain. The ultimate compressive strength of each gel was extracted after plotting the stress–strain curve for each sample. The ultimate compressive strength of a material is a bulk property of a material and the porosity of a scaffold directly tells about its strength.A number of factors like the inherent property of the monomer/polymer precursors, concentration of the precursors, concentration of the crosslinker, degree of crosslinking, type of crosslinking, synthesis temperature, etc. decide the mechanical fate of synthesized scaffolds. Thus, it becomes imperative to maintain equilibrium between the pore size and mechanical strength in order to achieve an ideal scaffold. Pore size and thickness of the pore walls together formulate the pore volume, which plays an important role in mechanical property of the scaffold.38 From the synthesis perspective, cryogels are flexible since they allow some degree of freedom to the developer in terms of porosity and mechanical strength. Often a scaffold cannot be used for a specific application due to its lacuna of pore size or the mechanical strength. Development of application specific matrices is easier with cryogels compared to other gels.
3.5 Swelling kinetics
The distribution of pores and degree of interconnectivity plays a pivotal role in the swelling kinetics of the gels. It can be clearly seen that cryogels swell instantaneously to their maximum limits (Fig. 12). This is an indirect indication towards how well the pores are organized in cryogels. This characteristic feature of cryogels can be beneficial in situations wherein retention of large volumes of nutrients is required in a shorter span of time. One of the possible reasons is the presence of highly interconnected capillary pores in cryogels.40 However, swelling also depends on concentration of the precursors and the pore size. Another important reason that might be responsible for a high swelling degree of cryogels is the presence of large pores. Hydrogels take more time to swell mainly due to the absence of capillary pores and that is the reason hydrogels swell at later time points. Moreover, due to low degree of interconnectivity of pores in hydrogels, there is a slow response shown by hydrogels when it comes to uptake of fluids. Due to well-defined, larger pores, all cryogels synthesized at −15 °C swell to their maximum value in a short span of time. However, pAAm cryogels, swell maximum out of all the gels synthesized at −15 °C, which may be attributed to their synthetic precursors. Cryogels synthesized at −5 °C follow the cryogels synthesized at −15 °C which is due to the cryo structuring when compared with both types of hydrogels.3.6 Percentage pore volume
The percentage pore volume of 72–92% was observed among fabricated gels using cyclo-hexane method. All the cryogels synthesized at −15 °C exhibit maximum pore volume that can be attributed to larger pores caused by the cryo-structuring. FD hydrogels seem to possess closed pores, which have also been seen in SEM images and because of this the hydrogels have less percentage pore volume. Also the pore volume in case of FD hydrogels is less while the thickness of the walls is more, this can also be another potent reason of less pore volume in hydrogels. The gels fabricated at −5 °C possess larger pores when compared to the hydrogels. Again cryo-treatment may be responsible for the apparent results. We also observed a decrease in the percentage porosity with the increase in concentration of the precursors. This decrease may be attributed to the comparatively smaller pores those are formed with the increasing concentration.3.7 Cell–material interaction
Significant differences in the mechanical and rheological properties of gels with variation in temperature motivated us to study the cell behaviour on them. We studied the behaviour of chicken chondrocytes on different cryogel matrices synthesized at −12 °C. Moreover, natural polymeric precursors were preferred over synthetic polymers in the cell material interaction section mainly due to higher affinity of cells to natural polymers than synthetic polymers. As CAG cryogels have already been used in our laboratory for cartilage tissue engineering applications we furthered our research on these matrices. We tried to evaluate the effect of mechanical and rheological behaviour of different materials on the growth and proliferation of chicken chondrocytes. CAG cryogels were chosen as a standard for chondrocyte proliferation while chitosan and chitosan–gelatin cryogels were used as controls to understand how cells responded on other material surfaces.The cell viability analysis results using the MTT assay were quite fascinating and unravelled expected results. While the chondrocytes proliferated at a faster rate on the 2D i.e. treated well plates, the proliferation rate reached its maxima at the second week after which the cells could not proliferate much and the cell number decreased to a lower value on the last time point. This can be due to the presence of lesser surface area available for the cells. So initially when the cells proliferate they occupy the available surface after which they die due to lack of surface and build up of the toxins (Fig. 13). Among the polymeric 3D scaffolds, chitosan cryogel scaffold exhibits maximum cell growth at the two-week time point. However, there is a sudden reduction in the proliferation that is possibly caused by the incompetence of the scaffold in terms of supporting chondrocytes due to abominable mechanical and elastic properties. Due to a balance of elastic and mechanical properties and optimum surface area, CAG scaffolds were expected to exude better results. The scaffolds show a gradual growth initially which maybe because the cells require some time to acclimatize to the 3D environment and a sustained cell growth is seen even on the later (3rd week) time point also. CAG scaffolds among all other scaffolds show maximum sustained growth and thus it can be concluded from the biocompatibility assay that all the scaffolds support the growth and proliferation of chicken chondrocytes. However, CAG scaffolds are the best among the group for the proliferation of chondrocytes due to the balance of pore size and rheological properties, which are pre-requisites for a tissue-engineering scaffold.
Once the chondrocyte viability and proliferation results were compared and analyzed, we chose to characterize the better scaffold in terms of mechanical and rheological properties and thus cell seeded CAG scaffolds were chosen. The mechanical study clearly indicates that there is a huge increase in the compressive strength of the scaffolds seeded with cells after four weeks. The ultimate compressive strength increases from a value of 50 kPa to a value of approximately 250 kPa (Fig. 15; bottom panel). The probable reason of this increase can be due to the secretion of extra cellular matrix (ECM) by the cells, which mainly comprises of strong collagen fibers. ECM secreted by the chondrocytes occupies the space between the pores of these cryogel scaffolds and may also integrate closely with the scaffold to increase their mechanical strength. On the contrary, the rheological results suggest that the overall elasticity of the material reduces after four weeks of cell growth when compared to the control sample and there is a minimal reduction in the viscous property of the seeded samples, which obviously happens due to the ECM secretions (Fig. 15; top panel). These results hold significant importance since these parameters are very important for a scaffold to qualify for cartilage tissue engineering applications. The above results along with the cell viability results show the potential of CAG scaffolds as the most appropriate scaffolds for cartilage tissue engineering.
4. Conclusion
Rheological and mechanical analysis performed on synthesized polymeric gels indicated influence of “fabrication temperature” on mechanical as well as visco-elastic behaviour of the material. Our results revealed effect of cryo-structuring on distribution of pores and pore morphology with respect to all crosslinking methods used for synthesis of polymeric gels. This was also evident from comparative analysis of FD hydrogels and conventional hydrogels against cryogels. Comparative study of hydrogels against cryogels reflected more pronounced effect of temperature on mechanical fate of scaffold. A decrease in temperature leads to the formation of smother and larger pores as a result of fast nucleation of ice crystals. Moreover, this decrease in synthesis temperature can also cause a decrease in compressive strength of a polymeric material owing to larger pores. Our results indicated a direct correlation of porosity with visco-elasticity. The effect of visco-elastic and mechanical properties on cell material interaction was also studied. Our study suggested that scaffolds demonstrating a right balance of bulk properties are thus good candidates for supporting cell proliferation and growth.Acknowledgements
The authors would like to acknowledge the Department of Biotechnology (DBT) and Department of Science and Technology (DST), Ministry of Science and Technology, Govt. of India. AK would like to acknowledge TATA Innovation Fellowship from DBT.Notes and references
- F. Rosso, A. Giordano, M. Barbarisi and A. Barbarisi, J. Cell. Physiol., 2004, 199, 174–180 CrossRef CAS PubMed.
- M. van Dijk, S. A. Göransson and S. Strömblad, Exp. Cell Res., 2013, 319, 1663–1670 CrossRef CAS PubMed.
- M. E. Lukashev and Z. Werb, Trends Cell Biol., 1998, 8, 437–441 CrossRef CAS.
- G. Pasquinelli, C. Orrico, L. Foroni, F. Bonafè, M. Carboni, C. Guarnieri, S. Raimondo, C. Penna, S. Geuna, P. Pagliaro, A. Freyrie, A. Stella, C. M. Caldarera and C. Muscari, J. Anat., 2008, 213, 520–530 CrossRef CAS PubMed.
- A. M. Pizzo, K. Kokini, L. C. Vaughn, B. Z. Waisner and S. L. Voytik-Harbin, J. Appl. Physiol., 2005, 98, 1909–1921 CrossRef CAS PubMed.
- E. Masaeli, M. Morshed, P. Rasekhian, S. Karbasi, K. Karbalaie, F. Karamali, D. Abedi, S. Razavi, A. Jafarian-Dehkordi, M. H. Nasr-Esfahani and H. Baharvand, J. Biomed. Mater. Res., Part A, 2012, 100, 1907–1918 CrossRef PubMed.
- C. B. Khatiwala, S. R. Peyton and A. J. Putnam, Am. J. Physiol.: Cell Physiol., 2006, 290, C1640–C1650 CrossRef CAS PubMed.
- Y. Sun, C. S. Chen and J. Fu, Annu. Rev. Biophys., 2012, 41, 519–542 CrossRef PubMed.
- A. F. Bayomy, M. Bauer, Y. Qiu and R. Liao, Life Sci., 2012, 91, 823–827 CrossRef CAS PubMed.
- A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Cell, 2006, 126, 677–689 CrossRef CAS PubMed.
- S. Peyton, Integr. Biol., 2012, 4, 37–52 RSC.
- S. Li, N. F. Huang and S. Hsu, J. Cell. Biochem., 2005, 96, 1110–1126 CrossRef CAS PubMed.
- W. Goldmann, Cell Biol. Int., 2012, 36, 567–570 CrossRef PubMed.
- P. Lu, V. Weaver and Z. Werb, J. Cell Biol., 2012, 196, 395–406 CrossRef CAS PubMed.
- M. Chiquet, L. Gelman, R. Lutz and S. Maier, Biochim. Biophys. Acta, 2009, 1793, 911–920 CrossRef CAS PubMed.
- M. A. Schwartz, Cold Spring Harbor Perspect. Biol., 2010, 2, a005066 CAS.
- J. P. Stegemann, H. Hong and R. M. Nerem, J. Appl. Physiol., 2005, 98, 2321–2327 CrossRef PubMed.
- S. A. Guelcher and J. A. Sterling, Cancer Microenviron., 2011, 4, 247–259 CrossRef CAS PubMed.
- W. Zhai, X. Lü, J. Chang, Y. Zhou and H. Zhang, Acta Biomater., 2010, 6, 389–395 CrossRef CAS PubMed.
- H. Geckil, F. Xu, X. Zhang, S. Moon and U. Demirci, Nanomedicine, 2010, 5, 469–484 CrossRef CAS PubMed.
- S. R. Peyton, C. B. Raub, V. P. Keschrumrus and A. J. Putnam, Biomaterials, 2006, 27, 4881–4893 CrossRef CAS PubMed.
- S. Dwivedi, P. Khatri, G. R. Mehra and V. Kumar, Int. J. Pharm. Biol. Arch., 2012, 2, 1588–1597 Search PubMed.
- T. Billiet, M. Vandenhaute, J. Schelfhout, S. Van Vlierberghe and P. Dubruel, Biomaterials, 2012, 33, 6020–6041 CrossRef CAS PubMed.
- C. Rivest, V. Yadav, D. Morisson, J. Rubin, B. Ni, A. Mahdavi, J. M. Karp and A. Khademhosseini, J. Mech. Mater. Struct., 2007, 2, 1103–1119 CrossRef.
- O. Okay, in Hydrogel Sensors and Actuators, ed. G. Gellach and K.-F. Arndt, Springer, Germany, 2010, pp. 1–14 Search PubMed.
- A. Kumar, A. Srivastava, I. Galaev and B. Mattiasson, Prog. Polym. Sci., 2007, 32, 1205–1237 CrossRef CAS PubMed.
- M. V. Dinu, M. M. Ozmen, E. S. Dragan and O. Okay, Polymer, 2007, 48, 195 CrossRef CAS PubMed.
- A. Kumar, R. Mishra, Y. Reinwald and S. Bhat, Mater. Today, 2010, 13, 42–44 CrossRef CAS.
- V. I. Lozinsky, Russ. Chem. Rev., 2002, 71, 489–511 CrossRef CAS PubMed.
- S. Bhat and A. Kumar, J. Postgrad. Med. Educ. Res., 2012, 46, 81–89 CrossRef.
- T. Vishnoi and A. Kumar, J. Mater. Sci.: Mater. Med., 2013, 24, 447–459 CrossRef CAS PubMed.
- S. Bhat, A. Tripathi and A. Kumar, J. R. Soc., Interface, 2011, 8, 540–554 CrossRef CAS PubMed.
- A. Kumar and A. Srivastava, Nat. Protoc., 2010, 5, 1737–1747 CrossRef CAS PubMed.
- S. Bhat, L. Lidgren and A. Kumar, Macromol. Biosci., 2013, 13, 827–837 CrossRef CAS PubMed.
- V. I. Lozinsky, L. G. Damshkaln, K. O. Bloch, P. Vardi, N. V. Grinberg, T. V. Burova and V. Y. Grinberg, J. Appl. Polym. Sci., 2008, 108, 3046–3062 CrossRef CAS.
- B. Amsden, Macromolecules, 1998, 31, 8382–8395 CrossRef CAS.
- J. Wolfe, G. Bryant and K. L. Koster, CryoLetters, 2002, 23, 157–166 Search PubMed.
- F. M. Plieva, M. Karlsson, M.-R. Aguilar, D. Gomez, S. Mikhalovsky and I. Y. Galaev, Soft Matter, 2005, 1, 303–309 RSC.
- S. B. Ross-Murphy, in Polymer Gels, ed. D. DeRossi, K. Kajiwara, Y. Osada and A. Yamauchi, Springer, Germany, 1991, pp. 21–39 Search PubMed.
- J. Yun, H. Kirsebom, I. Y. Galaev and B. Mattiasson, J. Sep. Sci., 2009, 32, 2601–2607 CrossRef CAS PubMed.
Footnote |
| † Authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |