Preparation and characterization of amphoteric polycarboxylate and the hydration mechanism study used in portland cement
Qinyu Rena,
Huawei Zou*a,
Mei Lianga,
Yongmei Wangb and
Jinchun Wangb
aState Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University, Chengdu 610065, China. E-mail: hwzou@163.com; liangmeiww@163.com; Fax: +86-28-85402465; Tel: +86-28-85408288
bSichuan Chang'an Yucai Jiancai Inc, Chengdu, China
First published on 15th August 2014
Abstract
A series of comb-like polymer, amphoteric polycarboxylic (APC), was designed as a dispersant for portland cement. APC was synthesized in aqueous solution by macromonomer of butenyl alkylene polyoxyethylene-polyoxypropylene ether (BAPP), sodium methacrylate (SMA), acrylic acid (AA) and [2-(methacryloyloxy)ethyl]trimethylammonium chloride solution (MAEAC). The molecule structure of amphoteric polycarboxylic dispersant was characterized by Fourier transformer infrared (FTIR), and the molecular weight of the APC was determined by gel permeation chromatography (GPC). The dispersion capacity of the APC in cement was also measured. The result shows that the amphoteric polycarboxylic dispersion agent is suitable for portland cement. The APC dispersant with both anionic group and cationic group will provide the better anchoring action with the cement via the adsorption of ions. The hydration mechanism of cement with a certain amount of APC is discussed. We found that the APC could affect the hydration process, which was performed through retarding the generation of ettringite and affecting the crystal enthalpy and its thermal stability in the hydrated product.
1. Introduction
Superplasticizers (SPs) are frequently used in concrete technology to improve the workability of concrete systems, leading to higher strength and better durability of the hardened products. Among these SPs, polycarboxylate (PC) has been in demand in recent years.1,2 The structure of PC admixtures consists of a linear hydrocarbon backbone with carboxylate and ether group side chains. Their adsorption on cement particles, mediated by their carboxylate groups, disperses the cement grains as a result of the steric repulsion generated by the long ether group chains.3–5 A PC acting as a new general water-reducing agent has many merits, such as low dosage, strong dispersing ability, good ability for keeping slump, good regulation and control of molecular structure and no formaldehyde is released in the production.The chemical structure of the superplasticizer's has considerable effect on their functional efficiency. According to Wei Fan et al.,6 a partial substitution of the carboxyl groups by trialkoxysilane in the polymer makes them more resistant to sulfate ions. They suggested that the high adsorption capacity of these SPs results from the formation of strong bonds between hydroxysilane groups and calcium silicate hydrate phases. However, an excess of silylated functions is detrimental for adsorption. According to the research from J. Plank et al.,7 modified PCEs possessing hydroxyl alkyl lateral chains were synthesized from methacrylic acid and hydroxy alkyl methacrylate esters (alkyl = ethyl, propyl and butyl), and were tested for their dispersion performance in cement in the absence and presence of montmorillonite clay. A novel PCE type was found to well disperse the cement, even when the clay was present. This research group found that the novel PCE only adsorbs on the surface of the clay and does not incorporate into the layer structure of the aluminosilicates. Shenghua Lv et al.8 synthesized PCs with ether linkages and ester linkages between the main chains and the poly(ethylene glycol) (PEG) branch chains. Their results showed that the linkage between main chains and PEG branch chains in PCs molecules had an important influence on the performance of the cement paste and concrete prepared from them.
In recent studies about the chemical structure modifications9 of PC-based SPs, modification attempts usually focus on the charge density differentiation, side chain length, and main backbone length, degree of backbone polymerization and composition of the functional groups.
In this study, we aimed to prepare a novel amphoteric polycarboxylate as a cement paste dispersant. With hydrogen peroxide and vitamin C as an oxidation–reduction catalyst system, we used macromonomer butenyl alkylene polyoxyethylene-polyoxypropylene ether (BAPP), sodium methacrylate (SMA), acrylic acid (AA) and [2-(methacryloyloxy)ethyl]trimethylammonium chloride solution (MAEAC) to synthesize the novel amphoteric polycarboxylic (APC) dispersant for portland cement. Our aim is to improve the compatibility of the PC superplasticizer in portland cement by introducing the cationic monomer. Superplasticizers may react with cement components10 and/or with the cement hydrated products11,12 and may also affect the cement's hydration kinetics and cement setting.13–16 Therefore, we discuss the effect of APC on the cement hydration mechanism using scanning electron microscopy (SEM), X-ray diffraction (XRD), differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA).
2. Experimental
2.1 Materials
[2-(Methacryloyloxy)ethyl]trimethylammonium chloride solution (MAEAC) was purchased from Aladdin Reagent. Butenyl alkylene polyoxyethylene-polyoxypropylene ether (BAPP) (double bond retention > 88%, Mw = 2250–2550) was supplied by Liaoning Oxichem Inc. Sodium methacrylate (SMA) was kindly supplied by Chang'an Yucai Inc. Acrylic acid (AA), hydrogen peroxide (H2O2), vitamin C (Vc) and other chemical reagents were purchased from Chengdu Kelong Inc. (Chengdu, China), and were used without further purification. Standard cement and sand was purchased from China Building Materials Academy. The chemical composition and the physical properties of the cement provided by the producer are shown in Table 1.| Oxide | % | Physical properties% | % |
|---|---|---|---|
| SiO2 | 25.2 | Fineness (0.08/%) | 1.7 |
| Al2O3 | 6.28 | Density (g cm−3) | 3.14 |
| Fe2O3 | 4.09 | Specific surface area (m2 kg−1) | 351 |
| CaO | 54.77 | Standard consistency (%) | 26.4 |
| MgO | 2.60 | Stability (mm) | 0.5 |
| SO3 | 2.63 | Setting time (min) | |
| Na2Oeq | 0.57 | Initial | 151 |
| f-CaO | 0.69 | Final | 215 |
| Loss | 2.48 | Flexural strength (MPa) | |
| CL− | 0.008 | 3 days | 5.0 |
| Total | 99.32 | 28 days | — |
| Compressive strength (MPa) | |||
| 3 days | 26.7 | ||
| 28 days | — |
2.2 Preparation of amphoteric polycarboxylate (APC)
A certain amount of BAPP and deionized water was fed into a 500 mL four-neck round-bottomed flask. AA and MAEAC were dissolved in some volume of water, which was labeled as solution A. Vc and SMA were also dissolved in a small amount of water, which was labeled as solution B. After bubbling with nitrogen for 30 min, the reaction mixture was allowed to warm to 70 °C under a nitrogen atmosphere. After the addition of H2O2 into the flask, solution A and solution B were added dropwise with an acceleration of 80 and 40 drops per min, respectively. The reaction was stopped after 6 h. The structure was confirmed by FTIR and GPC. The synthesis process of the APC is shown in Scheme 1 and the formula of the APCs is showed in Table 2.| Sample | BAPP (g) | AA (g) | MAEAC (g) | H2O2 (g) | Vc (g) | SMA (g) | H2O (g) |
|---|---|---|---|---|---|---|---|
| APC-1 | 48 | 4.32 | 4.154 | 1.5 | 0.236 | 0.5 | 154 |
| APC-2 | 48 | 4.32 | 2.077 | 1.5 | 0.236 | 0.5 | 154 |
| APC-3 | 48 | 8.64 | 4.154 | 1.5 | 0.236 | 0.5 | 154 |
2.3 Solid content
A weighing bottle was placed in an electrothermal thermostatic drying oven at 65 °C for 30 min, and then placed into a desiccator for cooling to room temperature. We repeated this procedure until the weight of the weighing bottle was constant. This weighing bottle was used to weigh about 2 g APC solution, accurate to 1 mg. We then placed the bottle in the oven at 65 °C. After drying for 24 h, we elevated the temperature to 105 °C and dried for another 4 h. This procedure was repeated until a constant weight was obtained. The solid content was calculated as follows:| ω = (m1 − m2)/(m1 − m0) × 100% | (1) |
Measurements were repeated three times, and the average values were obtained.
2.4 Mortar fluidity
The test was carried out according to GB.T2419-2005 produced by the Standardization Administration of the People's Republic of China. Fresh mortar pastes with APC were prepared according to the relevant provisions GB177. The mortar was made with a water/cement (w/c) ratio of 0.47, and then the APC was added into the mixing water at a solid dosage of 0.15%. The mixture was then placed aside for some time. In addition, mortar without APC was prepared with the same conditions. Alcohol was used to stop the hydration after 30 min and 24 h. The strength of the hydrated products was obtained by vacuum drying and then grinding.2.5 Testing methods
FTIR spectrum was obtained by a Fourier transform infrared spectrometer (Nicolet 570) using potassium bromide (KBr) pellets to investigate the structure of the APC. The spectra were recorded over the spectral range 400–4000 cm−1 at a resolution of 4 cm−1.The molecular weight was determined on a gel permeation chromatography (GPC, also known as size exclusion chromatography (SEC)) Waters 1525/2414 instrument (Waters, USA) with a differential refraction detector was used for the measurement of the molecular weight of the polymer. Sodium azide solution (0.1 mol L−1) was utilized as the carrying phase at a flow rate of 1 mL min−1, where large molecules pass the column more quickly than small molecules. The measurement was performed at 40 °C, using polyethylene glycol as the calibration standard.
The zeta potential of the cement suspension was measured using a Zetasizer Nano ZS (Malvern Instruments). The portland cement (0.25 g) was added to 50 mL water containing a certain amount of APCs. The cement suspension was allowed to stir for 3 min before the zeta potential measurement was performed. All the measurements were conducted at 25 °C. The mean value of the zeta potentials, which were measured three times, was used in this study.
The morphology of the fracture surfaces was observed using a scanning electron microscope (SEM: JSM-5900, JEOL Co., Ltd) at an accelerating voltage of 10 kV. Prior to examination, the surfaces were coated with a thin layer of gold to improve conductivity and prevent charging.
X-ray diffraction (XRD) patterns were recorded by monitoring the diffraction angle (2θ) from 2° to 70° on a D/MAX-III power diffractometer (DY1291, Philips, Holland) with a wavelength of 0.1542 nm of Cu Kα.
The hydration crystallization behavior was carried out by a differential scanning calorimeter (DSC204, Netzsch Com, Germany). Samples weighing around 12 mg were used for characterization. Nitrogen purge gas with a flux of 50 mL min−1 was used to prevent the thermal degradation of the samples during scanning. The samples were heated (at 10 °C min−1) from room temperature up to 350 °C.
The thermal stability was studied using thermogravimetric analysis (TGA: Q500, TA Co, Ltd. USA). The sample masses ranging from 5–10 mg were heated from 25 °C to 800 °C at a rate of 10 °C min−1. The experiments were performed under nitrogen atmosphere (at a flow rate of 100 mL min−1).
3. Results and discussion
3.1 Structure of APCs
Infrared spectra of the amphoteric polycarboxylate dispersant are depicted in Fig. 1. No double bond is formed in the molecules because there is no absorption peak in the rage of 2500–2000 cm−1. The peak at 1729 cm−1 belongs to COO−. The peak at 954 cm−1 is the definite vibration absorption peak of the quaternary ammonium ion –N+ (CH3)3. The peak at 1106 cm−1 confirms the existence of C–O–C.The above analysis results show that APC molecules consist of negative COO− group, large side chains of polyethylene glycol and positive group –N+(CH3)3.
GPC results are shown in Fig. 2 and Table 3. Peak 1 is the outflow of the APCs, whereas peak 2 belongs to the unreacted polyether macromonomer. The molecular weight of APC-3 was the highest, and APC-1 had the largest distribution. With the same other conditions, the reaction monomer of the APC-2 was the smallest; therefore, it has the lowest molecular weight. Correspondingly, APC-3 had a considerably higher molecule weight because of the double amount of AA. FTIR and GPC analyses show that the preparation of the APC was successful.
| Mw (× 105) | Mn (× 105) | Mw/Mn | |
|---|---|---|---|
| APC-1 | 7.80 | 1.83 | 4.25 |
| APC-2 | 4.96 | 3.44 | 1.44 |
| APC-3 | 26.7 | 9.85 | 2.71 |
3.2 Mortar fluidity at certain addition dosage
Fig. 3 shows the initial fluidity of the APCs with a w/c ratio of 0.47 and solid dosage of 0.15%. We can see that more carboxylic acid groups cause better mortar fluidity. The fluidities of the APC-1 and APC-2 are almost the same, which was difficult to explain the contribution of the cationic monomer superplasticizer. For further study, we carried out another experiment. Except that no MAEAC was added, the other experimental conditions were the same as for the preparation of APC-3. The mortar fluidity at solid dosage of 0.15% was 245 mm. The results indicate that the COO− plays a predominant role in the adsorption process on the cement particles. When COO− is sufficient, cations on the main chain can also contribute to the adsorption effect.The portland cement mainly consists of 3CaO·SiO2 (C3S), 2CaO·SiO2 (C2S), 3CaO·Al2O3 (C3A) and 4CaO·Al2O3·Fe2O3 (C4AF) (shown in Table 1). It was reported that C3A and C4AF were positive, whereas C3S and C2S were negative.17 The molecular model of the APC is shown in Scheme 1. Both the anionic groups and cationic groups exist in APC. The mechanism for adsorption is that anionic groups of the superplasticizer molecule are adsorbed on the surface of C3A and C4AF, whereas cationic groups are adsorbed on C3S and C2S. The APC dispersant with both anionic groups and cationic groups can provide better anchoring action of the cement via the adsorption of the ion pair. At the same time, APC molecules containing a certain amount of polyether inside chains can form stable adsorbed layers on the surface of the cement particles. When the polymer chains are thoroughly dissolved and appropriately unfolded in water, their steric hindrance can make the cement particles disperse more stably.
According to Kissa,18 from the viewpoint of thermodynamics, the efficiency of superplasticizers depends on their molecular weights. Superplasticizers with large molecular weights, because of greater contact by their molecules with cement particles' surface, provide higher affinity to the surface, leading to better paste fluidity than the low molecular weight polymers. The molecule weight of the APC-3 is the highest and the fluidity test result is consistent with Kissa's opinion.
Fig. 4 shows the flow of mortars and retention capability with APCs. It can be seen from Fig. 4 that all the three superplasticizers did not show good retention capability; the fluidity gradually decreased with prolonged time. The probable reason is that the hydration of the cement is process, while there is no effective group in the APCs to postpone the process.
 | ||
| Fig. 4 Fluidity vs. time of the cement paste with APC-1, APC-2, APC-3 (w/c = 0.47, solid dosage = 0.15%). | ||
3.3 Zeta potential of the cement suspension
The effects of the APC dispersant on the zeta potential of the portland cement are presented in Table 4. When cement and water form dispersion, its zeta potential has a positive value. With the APCs added, the zeta potential of the cement is negative and increases with increasing APCs. Therefore, the adsorbed amount of the dispersant on the surface of the cement particles increases. In the meantime, there are more COO− groups on the cement particle surface towards the water. This reduces the interaction between the cement particles, such that the whole system becomes more stable. The mean value of the APC-1 system is lower than that of both APC-2 and APC-3. The reason may be that APC-2 and APC-3 have more net negative charge; thus, the electrostatic repulsion is stronger, leading to better stability. As we can see from Table 4, when the dosage of APC changes from 0.12 to 0.16, the zeta potential of the APC-2 and APC-3 systems decrease a little. This is partly because the dispersant molecules could arrange in opposite directions when the adsorption of the dispersant arrives at the saturation value. The dispersant adsorption structure of the electrical double layer should be changed. Thus, the zeta potential of the cement slowly decreases.| Zeta potential (mV) | |||
|---|---|---|---|
| APC dosage | APC-1 | APC-2 | APC-3 |
| 0 | 0.167 | 0.167 | 0.167 |
| 0.04 | −2.32 | −3.54 | −3.3 |
| 0.08 | −2.46 | −3.61 | −3.48 |
| 0.12 | −2.55 | −3.74 | −3.67 |
| 0.16 | −2.68 | −3.31 | −3.55 |
 | ||
| Fig. 5 FTIR spectra of the cement hydrated products, (a) different APCs hydrated 24 h, (b) one APC hydrated 30 min and 24 h. | ||
From Fig. 5(b), there is no considerable difference among the three APC hydrated products. But we can see that the characteristic absorption bands of H2O after hydration for 24 h were considerably higher than that after 30 min. This phenomenon revealed that the hydration of the cement proceeded slowly and would experience a relatively long process.
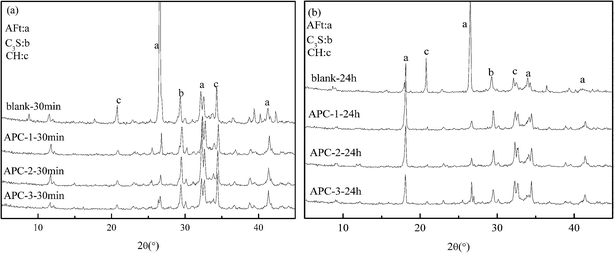
Fig. 7 shows the XRD of the hydrated products hydrated for 30 min and 24 h. The hydrated products AFt and CH can be detected by X-ray diffraction. We observed that there is a large difference between the blank sample and samples with the addition of APCs because APCs can retard the hydration of the cement. After hydration for both 30 min and 24 h, the formation of CH and AFt can be seen from the XRD. The AFt content in the blank is considerably higher than that in APCs, which was consistent with the results of SEM.
According to Fig. 8, there is an endothermic peak within a temperature range from 80–180 °C. The endothermic peak between 80–180 °C is mainly due to the loose of interlayer water in the calcium silicate gel and ettringite. It is noticeable that there are some differences between these peaks. The content of ions and molecular weight of the superplasticizer may result in the shift of the peaks. That is, different molecular structures lead to different hydration processes, thus, the differentiation of the crystal enthalpy is produced.
Samples with the addition of APCs have different weight losses in the first stage. The weight loss of APC-2 is greater than that of APC-1 and APC-3. The reason may be that APC-1 and APC-3 have longer molecular chains and cannot be completely wrapped inside ettringite crystals. A part of the molecular chain segment will stay outside and form colloidal with crystals. The colloidal is called the ettringite precursor and can be stabilized with organic molecules.
4. Conclusion
In this work, three kinds of APC were synthesized. FTIR and GPC confirmed that the polymerization was successful. The mortar fluidity indicated that the introduction of a cationic monomer in the main chain of PCs could increase the dispersing ability for the portland cement. The characterization of the hydrated product showed that APC would affect the hydration mechanism. XRD results showed that there was a large difference between the blank sample and samples with the addition of APC after 30 min hydration. The SEM measurements indicated that APC could retard the hydration process. DSC and TGA showed that molecular structures affected the hydration process. The crystal enthalpy of the AFt changed and thermal stability differed with different APCs.Acknowledgements
The authors would thank the Sichuan Chang'an Yucai Inc. for materials support and the Analytical and Testing Center of Sichuan University for providing SEM observation.References
- J. Plank and H. Bian, Cem. Concr. Res., 2010, 40, 710 CrossRef CAS PubMed.
- N. Mikanovic and C. Jolicoeur, Cem. Concr. Res., 2008, 38, 907 CrossRef CAS PubMed.
- A. Ohta, T. Sugiyama and Y. Tanaka, in 5th International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Rome, SP173, 1997, p. 359 Search PubMed.
- H. Uchikawa, S. Hanehara and D. Sawaki, Cem. Concr. Res., 1997, 27, 37 CrossRef CAS.
- K. Yamada, T. Takahashi, S. Hanehara and M. Matsuhisa, Cem. Concr. Res., 2000, 30, 197 CrossRef CAS.
- W. Fan, F. Stoffelbach, J. Rieger, L. Regnaud, A. Vichot, B. Bresson and N. Lequeux, Cem. Concr. Res., 2012, 42, 166 CrossRef CAS PubMed.
- L. Lei and J. Plank, Cem. Concr. Res., 2012, 42, 1299 CrossRef CAS PubMed.
- S. H. Lv, J. P. Duan, R. J. Gao, Q. Cao and D. Li, Polym. Adv. Technol., 2012, 23, 1596 CrossRef CAS.
- E. Janowska-Renkas, Construct. Build. Mater., 2013, 38, 1204 CrossRef PubMed.
- R. J. Flatt and Y. F. Houst, Cem. Concr. Res., 2001, 31, 1169 CrossRef CAS.
- J. Plank and C. Hirsch, Cem. Concr. Res., 2007, 37, 537 CrossRef CAS PubMed.
- W. Kurdowski, Stowarzyszenie Producentow Cementu, Cracow, 2010.
- C. M. Neubauer, M. Yang and H. M. Jennings, Adv. Cem. Based Mater., 1998, 8, 17 CrossRef CAS.
- S. Grzeszczyk and E. Janowska-Renkas, in XXXXVII Scientific Conference KILiW PAN and KN PZITB, Krynica, IV, 2001, p. 323.
- J. Stark, Cem. Concr. Res., 2011, 41, 666 CrossRef CAS PubMed.
- E. Janowska-Renkas, in Reology-theort and application, EKMA, Warsaw, 2011, p. 105 Search PubMed.
- K. Yoshioka, E.-I. Tazawa, K. Kawai and T. Enohata, Cem. Concr. Res., 2002, 32, 1507 CrossRef CAS.
- E. Kissa, Dispersions: Characterization, Testing and Measurement, Marcel Dekker, New York, 1999 Search PubMed.
- T. L. Hughes, C. M. Methven, T. G. J. Jones, S. E. Pelham, P. Fletcher and C. Hall, Adv. Cem. Based Mater., 1995, 2, 91 CrossRef CAS.
- S. N. Ghosh and S. K. Handoo, Cem. Concr. Res., 1980, 10, 771 CrossRef CAS.
- T. Matschei, B. Lothenbach and F. P. Glasser, Cem. Concr. Res., 2007, 37, 551 CrossRef CAS PubMed.
- H.-J. Kuzel, Cem. Concr. Compos., 1996, 18, 195 CrossRef CAS.
- J. Björnström, A. Martinelli, A. Matic, L. Börjesson and I. Panas, Chem. Phys. Lett., 2004, 392, 242 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |