One-pot green synthesis of β-artemether/arteether†
Atul Kumar* and
Ajay Kumar Bishnoi
Medicinal and Process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031, India. E-mail: dratulsax@gmail.com; Fax: +91-522-26234051; Tel: +91-522-2612411
First published on 26th June 2014
Abstract
An efficient one pot green synthesis of β-artemether/arteether from artemisinin has been developed using a sodium borohydride-cellulose sulfuric acid (CellSA) catalyst system. The green methodology is high yielding and the catalyst has good recyclability.
Introduction
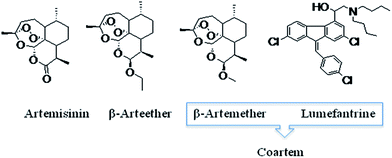
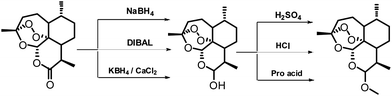
Malaria is a major tropical parasitic disease affecting 500 million people worldwide and causing the death of 1–2 million people per year, mostly children in Africa.1 Plasmodium is the causative agent of malaria; the most dangerous species is Plasmodium falciparum, which is responsible for malaria complications such as cerebral malaria. Artemisinin is a naturally occurring sesquiterpene lactone of prominent value in the pharmacological treatment of human malaria. Artemisinin and its derivatives (dihydroartemisinin, artemether, artesunate) are essential to modern malaria therapy, thus requiring an efficient synthetic route for these compounds (Fig. 1).2 Recently, the World Health Organization (WHO) has approved artemisinin combination therapy (ACT) containing artemether–lumefantrine (Coartem and Riamet, Falcynate-LF) and is a first-line treatment for uncomplicated Plasmodium falciparum malaria in most malaria-endemic countries.3Over the last decade, extensive synthetic efforts have been directed towards the development of synthesis for artemether/arteether. The synthesis of artemether/arteether from artemisinin involves two steps. First step involves the reduction of carbonyl group and in the second step etherification take place. These reported methodologies produce good yields but have some limitations such as a the carcinogenic solvents like benzene, use of highly hazardous Lewis acid and pro acid, use of column chromatograph in the separation of desired β-isomer and two step synthesis (Fig. 2). Lewis acid such as BF3·Et2O, Me3SiCl has the additional drawback of moisture sensitivity (Fig. 2).
Hence, the development of simple, and environmentally benign and sustainable resources protocol for the synthesis of artemether/arteether in high purity and yield with minimal side product formation such as anhydroartemisinin, 9-epi-artemether (α + β) is highly desirable.
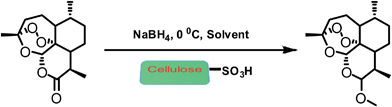
In this context, we wish to report here one-pot, environment friendly and cost effective process for preparation of methyl/ethyl ether derivative of artemisinin. The process consists of reduction of artemisinin to dihydroartemisinin and its in situ conversion to the desired ether derivative by the addition of an appropriate alcohol catalysed by combination of NaBH4/cellulose sulfuric acid (CellSA). The cellulose sulfuric acid is a natural biodegradable solid support acid catalyst,4 which efficiently promote the acid-catalyzed reactions and has cost effectively and good recyclability (Scheme 1).
Industries prefer reaction protocols that give the product just by filtration with no aqueous workup using hazardous organic solvents. The synthesis of artemether from artemisinin basically have reduction and etherification steps.5 Reductions step are ubiquitous in artemisinin to dihydroartemisinin due to their synthetic utility and high selectivity. Sodium borohydride (NaBH4) is used as reducing agent, followed by a multistep workup procedure. Diisobutylaluminium hydride (DIBAL-H) is another reducing agent, requiring dry dichloromethane as solvent, lower reaction temperature −78 °C and with a smaller yield than NaBH4.6
Recently, J. M. Williams et al. demonstrated a continuous flow chemistry protocol for stoichiometric reductions of artemisinin to dihydroartemisinin.7 There are several methods are reported by using acid catalysts such as sulphuric acid,8 phosphoric acid, anhydrous hydrochloric acid,9 p-toluenesulfonic acid,10 BF3·Et2O,11 Me3SiCl,12 AlCl3, or a cation exchange resin13 and pro acid14 like acetyl chloride, methane sulphonyl chloride, thionyl chloride etc. for the preparation ether derivative of artemisinin. The effort has been also focused to minimise the amount of acid catalysts and with the used of trialkyl orthoformate as a cosolvent. All these methodologies require use of hazardous reaction conditions in terms of solvent and catalysts. In this context the present report demonstrates the reduction of artemisinin and subsequent etherification in one pot in the presence of a catalytic amount of cellulose sulfuric acid (CellSA) to achieve good yields with excellent diastereoselectivity (β/α, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Result and discussion
Reduction of artemisinin with NaBH4
We began our study to screen the optimal reaction conditions for the synthesis of arteether from artemisinin. We first examined method for the preparation of arteether from artemisinin in one pot in just about 2.5 hours. In an experiment, NaBH4 (67 mg, 1.77 mmol, 2.5 equ.) was added over 10 min to a stirred mixture of artemisinin (200 mg, 0.71 mmol) and cellulose sulfuric acid (0.015 g), trimethylorthoacetate (0.5 ml), in EtOH (15 ml) and the solution was stirred at −5 to 0 °C for 60 min, and then stirred at room temperature for 1.5 h. During the investigations different amounts of NaBH4 were tested in order to find the smallest necessary amount for the best yield. Here, we were used 1.5, 2.0, 2.5 and 3.0 equivalent of NaBH4 as reducing agent in reaction media. Notably, we observed that 2.5 equ. of NaBH4 was efficient amount for better yield. The reaction solvent consisted of the respective alcohol supplemented with the corresponding trialkyl orthoacetate. The use of cosolvent such as trialkylorthoacetate was claimed to allow use of a lower amount of acid and to increase the reaction rate with high selectivity of β-arteether.15 The reaction was monitored by TLC and HPLC to check completion of the reaction. The cellulose sulfuric acid was removed by filtration, the filtrate was concentrated. Then we added a solution of sodium bicarbonate to terminate the reaction. The reaction mixture was vacuum evaporated to dryness. As simple filtration is enough to remove the catalyst and hence quench the reaction rather than the generally used aqueous workup, the protocol may find application in large-scale synthesis of artemether/arteether. Afterwards water was added to reaction mixture below 20 °C to precipitate out the crude arteether. The reaction mass was filtered and washed with ethanol. The reaction mass was heated to 40 ± 5 °C in water. The reaction mass was seeded with pure β-arteether. Then it was filtered, washed with chilled 50% solution of ethanol in water and suck dried. By above recrystallization method which removed the side product impurities such as anhydroartemisinin, 9-epi-arteether (α + β).16 A pure β-arteether obtained by crystallization method as given above and characterized by chromatogram of HPLC.Reduction of artemisinin with LiBHEt3
To examine and explore the of the solid support acid catalyst, cellulose sulfuric acid along with other reducing agent such as LiBHEt3. Of interest, we found the good yield of arteether with LiBHEt3/CellSA. Artemisinin was dissolved in dry THF and then cellulose sulfuric acid (0.015 g), trimethylorthoacetate (0.5 ml), EtOH (15 ml) was added and maintain the temperature to 0 °C for 60 min along with slow addition of LiBHEt3 solution (1 M in THF). After that reaction mixture was stirred at room temperature for a further 90 min. and work up with same methodology as mentioned above. The reaction took almost the same time and less yield than NaBH4 when LiBHEt3 use as reducing agent.HPLC was carried out on a C18 column (250 mm, 4 mm, and 5 μm) (Merck). The injection volume was 20 μl and the column effluent was monitored at 215 nm. The mobile phase consisted of acetonitrile and water, at a flow rate of 1 ml min−1. The optimized mobile phase was composed of 70% acetonitrile and 30% water. The detection was performed at 216 nm and the injection volume was 20 μl (Fig. 4 and 5).
The catalyst loading was optimized by synthesis of arteether as an model reaction. Reaction took place in 2.5 h with 0.015 g of catalyst loading and almost 82% yield was obtained. Lowering the catalyst loading to 0.005–0.01 g results in 75–78% yield of arteether with a longer reaction time of more than 2.5 h, whereas increasing the concentration of catalyst has no significant effect on the yield of the reaction. Thus our present study enabled us to find the efficient amount of catalyst which is summarized in Fig. 3.
Reusability of solid acid catalyst is address the green methodology of the reaction. Reusability of catalyst was observed under same reaction condition as given above, that is, synthesis of arteether. After completion of reaction, cellulose sulfuric acid was recovered from the reaction mixture by simple filtration, washed properly with acetone, and dried in oven for 3 h at 70 °C prior to its use in the absence of fresh catalyst. It was noticed that catalyst exhibited quite good reusability at least four additional times in subsequent reactions under the same reaction conditions without any remarkable loss in productivity (Fig. 3).
Conclusion
In summary, we have developed a green one pot synthetic methodology for the synthesis β-artemether/arteether using sodiumborohydride and cellulose sulfuric acid a natural carbohydrate solid acid catalyst system.This process requires no column chromatography with simple work-up, and it generates less hazardous waste. The developed methodology is efficient, cost-effective and the catalyst used is eco-friendly, reusable, and biodegradable.
Experimental section
Representative procedure for catalyst preparation
General procedure for the arteether from artemisinin in one-pot
To a solution of artemisinin (200 mg, 0.71 mmol) in ethanol (15 ml) and trimethyl orthoacetate (0.5 ml) was added NaBH4 (67 mg, 1.77 mmol, 2.5 equ.) and cellulose sulfuric acid (0.015 g). Reaction mixture was carried out at −5 to 0 °C for 60 min, and then stirred at room temperature for 1.5 h. Then we added a solution of sodium bicarbonate to quenched the reaction. The slurry was stirred in an below 20 °C for 1 h and allowed to settle for 30 min. Solid crude arteether was collected by filtration, and the cake was washed with of ethanol. The reaction mass was heated to 40 ± 5 °C in water. The reaction mass was seeded with pure β-arteether. Then it was filtered, washed with chilled 50% solution of ethanol in water and dried.General procedure for the artemether from artemisinin in one-pot
Artemisinin (200 mg, 0.71 mmol) in methanol (15 ml) and trimethylorthoformate (0.5 ml), cellulose sulfuric acid (0.015 g), was carried out at −5 to 0 °C for 60 min, and then stirred at room temperature for 1.5 h. The reaction was monitored by TLC and HPLC to check completion of the reaction. The cellulose sulfuric acid was removed by filtration, the filtrate was concentrated. Then we added a solution of sodium bicarbonate to terminate the reaction. Then, follow above recrystallization method.Acknowledgements
A.K.B. is thankful to CSIR-SRF New Delhi for financial support. The authors also acknowledge the analytical facilities of CSIR-CDRI. Financial support from CSIR-Network project BSC-0104. The CSIR-CDRI Communication No. is 8732.Notes and references
-
(a) World Health Organization, 10 Facts on Malaria, http://www.who.int/features/factfiles/malaria Search PubMed;
(b) C. J. L. Murray, L. C. Rosenfeld, S. S. Lim, K. G. Andrews, K. J. Foreman, D. Haring, N. Fullman, M. Naghavi, R. Lozano and A. D. Lopez, Lancet, 2012, 379, 413–431 CrossRef
.
-
(a) D. L. Klayman, Science, 1985, 228, 1049 CAS
; (b) X. D. Luo and C. C. Shen, Med. Res. Rev., 1987, 7, 29–52 CrossRef CAS
; (c) A. Gulland, et al., BMJ [Br. Med. J.], 2012, 344, 895 CrossRef PubMed
; (d) C. J. L. Murray, L. C. Rosenfeld, S. S. Lim, K. G. Andrews, K. J. Foreman, D. Hqqaring, N. Fullman, M. Naghavi, R. Lozano and A. D. Lopez, Lancet, 2012, 379, 413–431 CrossRef
.
-
(a) C. Falade and C. Manyando, Malar. J., 2009, 8, 1–14 CrossRef PubMed
; (b) G. O. Adjei, J. A. L. Kurtzhals, O. P. Rodrigues, M. Alifrangis, L. C. G. Hoegberg, E. D. Kitcher, E. V. Badoe, R. Lamptey and B. Q. Goka, Malar. J., 2008, 7, 127 CrossRef PubMed
; (c) F. T. Aweeka and P. I. German, Clin. Pharmacol., 2008, 47, 91–102 CrossRef CAS PubMed
; (d) N. Tangpukdee, S. Krudsood, S. Srivilairit, N. Phophak, P. Chonsawat, W. Yanpanich, S. Kano and P. Wilairatana, J. Parasitol., 2008, 46, 65–70 Search PubMed
.
-
(a) K. Wilson and J. H. Clark, Pure Appl. Chem., 2000, 72, 1313–1319 CrossRef CAS
; (b) R. Breslow, Acc. Chem. Res., 1980, 13, 170 CrossRef CAS
; (c) S. Ahmad, R. Abbas and B. Zahra, Catal. Commun., 2008, 9, 13 CrossRef PubMed
; (d) S. Ahmad, M. Ali, R. Jafar and S. Ebrahim, Chem. Pharm. Bull., 2007, 55, 957–958 CrossRef
; (e) A. Kumar, S. Srivastava and G. Gupta, Tetrahedron Lett., 2010, 51, 517–520 CrossRef CAS PubMed
.
- R. W. Stringham and D. S. Teager, Org. Process Res. Dev., 2012, 16, 764–768 CrossRef CAS
.
-
(a) G. H. Posner, I. H. Paik, S. Sur, A. J. Mcriner, K. Borstnik, S. Xie and T. A. Shapiro, J. Med. Chem., 2003, 46, 1060–1065 CrossRef CAS PubMed
; (b) J. Ma, E. Katz, D. E. Kyle and H. Ziffer, J. Med. Chem., 2000, 43, 4228–4232 CrossRef CAS PubMed
; (c) M. A. Mitchell, S. Mehrotra, T. L. Johnson, J. D. Bonk, J. A. Vroman and R. Miller, J. Med. Chem., 1996, 39, 4149–4155 CrossRef PubMed
.
- F. Xiaolei, S. Victor, Y. Polina, D. P. Dorota, W. Jonathan and L. Alexei, Org. Process Res. Dev., 2012, 16, 1039–1042 CrossRef
.
- M. S. Degani, S. S. Narkhede, Y. Y. Pedgaonkar and S. S. Chavan, International Patent WO 2008087666A1, 2008
.
- M. Boehm, P. C. Fuenfschilling, M. Krieger, E. Kuesters and F. Struber, Org. Process Res. Dev., 2007, 11, 336–340 CrossRef CAS
.
- F. S. El-Feraly, M. A. Al-Vahya, K. V. Oribi, D. R. McPhail and A. T. McPhail, J. Nat. Prod., 1992, 55, 878–883 CrossRef CAS
.
-
(a) A. J. Lin, D. L. Klayman and W. K. Milhous, J. Med. Chem., 1987, 30, 2147–2150 CrossRef CAS
; (b) A. Brossi, B. Venugopalan, L. Dominguez Gerpe, H. J. C. Yeh, J. L. Flippen-Anderson, P. Buchs, X. D. Luo, W. Milhous and W. Peters, J. Med. Chem., 1988, 31, 645–650 CrossRef CAS
.
- R. S. Bhakuni, D. C. Jain and R. P. Sharma, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1995, 34B, 529–530 CAS
.
- R. S. Bhakuni, T. Singh, A. P. Kahol, A. Tewari, S. Tandon and S. P. S. Khanuja, Single pot conversion of artemisinin into artemether, US Pat. US6683193 B2, 2004 Search PubMed
.
- C. P. Bapat, S. J. Dheer and S. K. Dhamale, WO2009109989A1, 2009
.
-
(a) L. I. Jinliang, Z. Nan, J. Fei, Z. Yuqing and Y. Xiaoguang, WO2013040750A1, 2013
; (b) R. W. Stringham and D. S. Teager, Org. Process Res. Dev., 2012, 16, 764–768 CrossRef CAS
.
-
(a) A. J. Lin, A. D. Theoharides and D. L. Klayman, Tetrahedron, 1986, 42, 2181 CrossRef CAS
; (b) L. Dhooghe, S. Van Miert, H. Jansen, A. Vlietnck and L. Pieters, Pharmazie, 2007, 62, 900 CAS
; (c) R. K. Haynes, H. W. Chan, C. M. Lung, N. C. Ng, H. N. Wong, L. Y. Shek, I. D. Williams, A. Cartwright and M. F. Gomes, ChemMedChem, 2007, 2, 1448 CrossRef CAS PubMed
; (d) W. Cabri, A. Ciogli, I. D'Acquarica, M. Di Mattia, B. Galletti, F. Gasparrini, F. Giorgi, S. Lalli, M. Pierini and P. Simone, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2008, 875, 180 CrossRef CAS PubMed
; (e) Q. Haiyan, B. C. Kathrine, C. F. Xavier, T. Fang, R. Jukka and P. C. Lars, Org. Process Res. Dev., 2010, 14, 585–591 CrossRef
.
-
(a) A. Kumar, S. Srivastava and G. Gupta, Tetrahedron Lett., 2010, 51, 517–520 CrossRef CAS PubMed
; (b) A. Kumar, G. Gupta and S. Srivastava, J. Comb. Chem., 2010, 12, 458–462 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra of β-artemether/β-arteether and other details. See DOI: 10.1039/c4ra05531d |
| This journal is © The Royal Society of Chemistry 2014 |