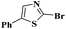
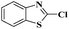
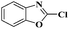
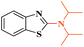
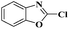
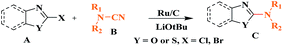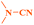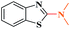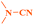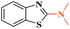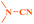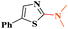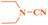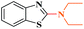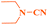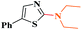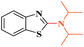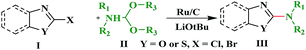Ru/C: a simple heterogeneous catalyst for the amination of azoles under ligand free conditions†
K. Harsha Vardhan Reddy,
B. S. P. Anil Kumar,
V. Prakash Reddy,
R. Uday Kumar and
Y. V. D. Nageswar*
MCP Division, Indian Institute of Chemical Technology, Hyderabad 500607, India. E-mail: dryvdnageswar@gmail.com; Fax: +91-40-27160512
First published on 1st September 2014
Abstract
A ligand free Ru/C-catalyzed amination of 2-halo azoles with a broad scope of aminating reagents has been developed. A variety of 2-aminoazole derivatives were synthesized in moderate to good yields by utilizing this protocol. The methodology is operationally simple and not sensitive to air and moisture. It provides potentially useful products by using an inexpensive and recyclable catalytic system under ligand free conditions without significant loss of its catalytic activity up to four cycles.
Introduction
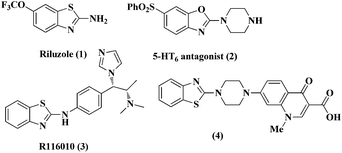
The discovery of mild and versatile methods for the formation of C–N bonds via cross-coupling reactions to synthesize pharmacologically active products represents a potential research domain. Aromatic nitrogen heterocyclic derivatives are ubiquitous structural motifs appearing in numerous biologically interesting natural products, as well as compounds of agrochemical and material sciences.1 The continued research in C–N coupling reactions produced an array of interesting approaches including the Ullmann coupling2 and Buchwald–Hartwig reactions.3In recent years, the synthesis of 2-aminoazoles has gained considerable attention in synthetic organic chemistry as functionalized azoles are valuable structural scaffolds4 and show great potential in the development of novel therapeutics.5,6 For example, Riluzole (1) is employed for the treatment of amyotrophic lateral sclerosis.7 5-HT receptors (2) (5-HT = 5-hydroxytryptamine, serotonin) are targets for the treatment of Alzheimer's disease and schizophrenia.8 R116010 (3) acts as an anticancer drug9 and N-disubstituted-2-aminobenzothiazole (4) is used as anti-HIV agent10 (Fig. 1).
In the view of the importance of 2-aminoazoles several procedures have been developed for the amination of azoles under the catalysis of transition metals, such as catalysts of Pd,11 Cu,12 Fe,13 Ni,14 Ag15 and Sc16 and their salts, as well as metal free conditions.17 However, these catalyst systems are usually not recyclable, expensive and moisture sensitive, thereby reducing the turn over number (TON) or turn over frequencies (TOF), which are significant from an industrial point of view.
Recently, our research group reported a green protocol for the synthesis of 2-N-substituted benzothiazoles by using nano copper oxide as a recyclable catalyst.18
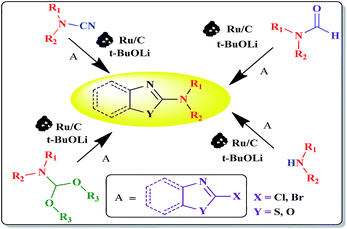
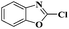
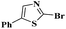
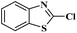
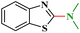
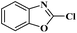
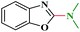
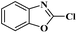
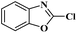
Ru/C has emerged as an important heterogeneous catalyst in synthetic organic chemistry. However, only few reports are found in the literature on charcoal-supported ruthenium catalysis.19 To the best our knowledge, there is no research report on the amination of 2-haloazoles with formamides/cyanamides/N,N-DMF di-alkyl acetal/amines catalyzed by recyclable Ru/C. In continuation of our interest in the field of transition metal catalyzed cross-coupling reactions,20 herein, we report a recyclable Ru/C-catalytic system for the amination of readily available 2-haloazoles under the influence of aminating reagents and LiOtBu as a base (Scheme 1).
Results and discussion
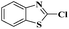
As a model reaction, initially the amination of 2-chloro benzothiazole (1.0 mmol) was investigated in DMF (2 mL) by using 5 wt% Ru/C (20 mg) under various conditions (Table 1). Among the different bases examined KOH, Et3N, Na2CO3, Cs2CO3, and K3PO4 decreased the yields (Table 1, entries 1–5). Whereas other bases, such as NaOtBu and KOtBu, provided the desired cross coupling products in moderate yields (Table 1, entries 6 and 7) However with LiOtBu, the product was obtained in maximum yield (Table 1, entry 8). The control experiments revealed that in the absence of either catalyst or the base the reaction did not proceed (Table 1, entries 11–12). The reaction is also very sluggish at room temperature (Table 1, entry 9). Nevertheless, a high yield was obtained when the reaction was conducted at 80 °C (Table 1, entry 8). Increasing the reaction time to 12 h did not improve the yield of the product (Table 1, entry 10). After screening several parameters, it was concluded that the desired cross-coupling product was formed in a high yield when 2-chlorobenzothiazole (1.0 mmol) was aminated in the presence of LiOtBu (1.0 mmol) and DMF (2 mL) when 5 wt% Ru/C (20 mg) catalyst was used at 80 °C.| Entry | Base (equiv.) | Temp. (°C) | Time (h) | Yieldb [%] |
|---|---|---|---|---|
| a Reactions were carried out with 2-chlorobenzothiazole (1.0 mmol), base (1.0 mmol), ruthenium 5 wt% on carbon (20 mg) and DMF (2 mL).b Isolated yield of the pure product.c Absence of catalyst.d Absence of base. | ||||
| 1 | KOH | 80 | 8 | 28 |
| 2 | Et3N | 80 | 8 | 19 |
| 3 | Na2CO3 | 80 | 8 | 40 |
| 4 | Cs2CO3 | 80 | 8 | 74 |
| 5 | K3PO4 | 80 | 8 | 40 |
| 6 | NaOtBu | 80 | 8 | 51 |
| 7 | KOtBu | 80 | 8 | 62 |
| 8 | LiOtBu | 80 | 8 | 90 |
| 9 | LiOtBu | rt | 8 | 25 |
| 10 | LiOtBu | 80 | 12 | 93 |
| 11 | LiOtBu | 80 | 8 | 0c |
| 12 | — | 80 | 8 | 0d |
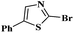
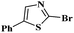
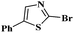
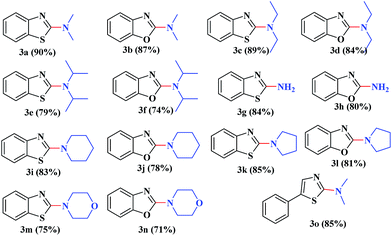
Under the optimized experimental conditions, the reaction of 2-halobenzothiazole and benzoxazole derivatives with different formamides produced the desired cross coupling products in good yields (Table 2). The reaction of 2-halobenzothiazole and benzoxazole with N,N-dimethylformamide, N,N-diethylformamide and N,N-di-tert-butylformamide obtain the desired cross coupling products in excellent yields (Table 2, entries, 3a–3f). Interestingly, formamide reacted with 2-chloro benzothiazole and benzoxazole to give the corresponding coupling products in excellent yields (Table 2, entries, 3g and 3h). The formamide derivatives with piperidine, pyrrolidine and morpholine systems were reacted with 2-halobenzothiazole and benzoxazoles to obtain the corresponding 2-aminated azoles in high yields (Table 2, entries, 3i–3n). 2-Bromo-4-phenylthiazole reacted with DMF to obtain the desired product in good yield (Table 2, entry, 3o).
| a Reaction conditions: azole (1.0 mmol), LiOtBu (1.0 mmol), ruthenium 5 wt% on carbon (20 mg), formamides (2 mL) at 80 °C for 8 h. |
|---|
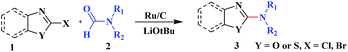 |
 |
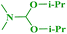
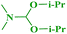
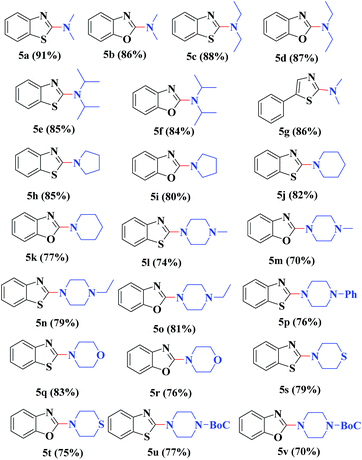
Under similar reaction conditions, this methodology was observed to be compatible with a broad range of aliphatic and cyclic amines with both 2-halobenzothiazoles and benzoxazoles. Aliphatic acyclic amines, such as dimethyl (5a, 5b and 5g), diethyl (5c and 5d), and diisopropyl (5e and 5f), amines, underwent smooth conversion to the desired products with excellent yields. In addition, other cyclic secondary amines, such as pyrrolidine (5h and 5i), piperidine (5j and 5k), 1-methylpiperazine (5l and 5m), 1-ethylpiperazine (5n and 5o), 1-phenylpiperazine (5p), morpholine (5q and 5r), thiomorpholine (5s and 5t), 1-boc-piperazine (5u and 5v), were also coupled with 2-halobenzothiazoles and benzoxazoles to obtain the corresponding 2-aminated azoles in good to excellent yields (Table 3).
| a Reaction conditions: azole (1.0 mmol), LiOtBu (1.0 mmol), ruthenium 5 wt% on carbon (20 mg), amines (2 mL) at 80 °C for 8 h. |
|---|
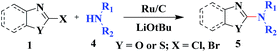 |
 |
As shown in Table 4, several 2-haloazoles were successfully reacted with different cyanamides, such as methyl, ethyl and isopropyl cyanamides, to provide the corresponding products in moderate to good yields under the optimized experimental reaction conditions (Table 4, entries 1–8).
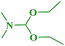
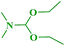
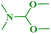
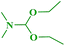
In continuation of our interest, N,N-dimethylbenzo[d]azol-2-amines were also synthesized by reacting 2-haloazoles with different N,N-DMF di-alkyl acetal derivatives to obtain the expected products in good yields (Table 5).
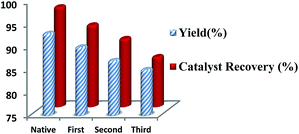
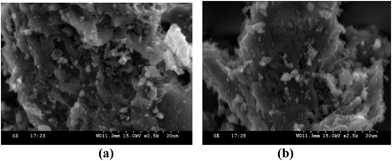
After the completion of the reaction, the Ru/C catalyst was separated by centrifugation and washed with ethyl acetate followed by acetone, and left to dry in a hot air oven. The dried Ru/C was reused directly for the next reaction cycle. No significant loss of catalytic activity was observed up to four cycles (Fig. 2). This was confirmed by SEM analysis (Fig. 3). A comparative SEM analysis study of the fresh catalyst and the recovered catalyst after four cycles confirmed that shape and size of the particles remain unchanged and this supports the assumption that the morphology of the catalyst remains the same even after recycling.
Conclusions
In summary, we have demonstrated the efficiency of the recyclable Ru/C as a catalyst in a novel protocol for the preparation of various substituted 2-aminated azoles via cross coupling of various 2-chloroazoles with formamides, amines, cyanamides and N,N-DMF di-alkyl acetals. This facile approach provides a green and practical methodology for the synthesis of 2-aminated azole derivatives. The recyclability and low cost of the catalyst system, compatibility of different amine sources, short reaction time, wide substrate scope and excellent product yields make this approach attractive.Experimental section
General experimental procedure for the amination of 2-chlorobenzothiazole
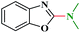
The reaction was carried in a 25 mL round bottom flask equipped with a magnetic stir bar charged with 2-chlorobenzothiazole (1 mmol), aminating agent (2 mL), LiOtBu (1.0 mmol), and Ru/C (20 mg) catalyst. The resulting reaction mixture was stirred at 80 °C for 8 h. The reaction progress was monitored by TLC. After the reaction, Ru/C was separated by centrifugation, the solvent was evaporated and the crude product was purified by column chromatography. The identity and purity of the product was confirmed by 1H NMR, 13 C NMR and ESI-MS.N,N-Dimethylbenzo[d]thiazol-2-amine (3a)
1H NMR (300 MHz, CDCl3): δ 7.60–7.55 (m, 2H), 7.32–7.25 (m, 1H), 7.07–7.02 (m, 1H), 3.19 (s, 6H); 13C NMR (75 MHz, CDCl3): 168.7, 153.2, 131.1, 130.0, 127.9, 125.9, 120.7, 118.7, 40.1. ESI-MS: m/z 179 [M + 1].N,N-Dimethylbenzo[d]oxazol-2-amine (3b)
1H NMR (300 MHz, CDCl3): δ 7.35 (d, J = 7.7 Hz, 1H), 7.23 (d, J = 7.9 Hz, 1H), 7.15–7.12 (m, 1H), 7.0–6.97 (m, 1H), 3.18 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 163.0, 148.9, 143.6, 123.8, 120.2, 115.9, 108.5, 37.6; ESI-MS: m/z 163 [M + 1].N,N-Diethylbenzo[d]thiazol-2-amine (3c)
1H NMR (300 MHz, CDCl3): δ 7.53 (t, J = 8.6 Hz, 2H), 7.25–7.22 (m, 1H), 7.01–6.96 (m, 1H), 3.48–3.42 (m, 4H), 1.19 (t, J = 7.2 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 166.8, 152.9, 130.2, 125.3, 120.2, 120.1, 118.1, 44.9, 12.4; ESI-MS: m/z 207 [M + 1].N,N-Diethylbenzo[d]oxazol-2-amine (3d)
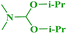
1H NMR (300 MHz, CDCl3): δ 7.35 (d, J = 7.9 Hz, 1H), 7.23 (d, J = 7.9 Hz, 1H), 7.13 (t, J = 7.7 Hz, 1H), 6.96 (t, J = 7.7 Hz, 1H), 3.55 (q, J = 7.1 Hz, 5H), 1.25 (t, J = 7.1 Hz, 8H); ESI-MS: m/z 191 [M + 1].N,N-Diisopropylbenzo[d]oxazol-2-amine (3f)
1H NMR (300 MHz, CDCl3): δ 7.35 (d, J = 7.7 Hz, 1H), 7.25 (d, J = 7.5 Hz, 1H), 7.13 (t, J = 7.6 Hz, 1H), 6.96 (t, J = 7.6 Hz, 1H), 4.28–4.15 (m, 2H), 1.38 (d, J = 6.9 Hz, 12H); 13C NMR (75 MHz, CDCl3): δ 162.2, 148.4, 142.8, 123.5, 119.5, 115.4, 108.2, 47.5, 20.7; ESI-MS: m/z 219 [M + 1].Benzo[d]thiazol-2-amine (3g)
1H NMR (300 MHz, CDCl3): δ 7.55 (d, J = 7.93 Hz, 1H), 7.496 (d, J = 7.93 Hz, 1H), 7.32–7.26 (m, 1H), 7.14–7.08 (m, 1H); 13C NMR (75 MHz, CDCl3): δ 166.6, 151.4, 130.9, 125.9, 122.1, 120.8, 118.6; ESI-MS: m/z 151 [M + 1].2-(Piperidin-1-yl)benzo[d]thiazole (3i)
1H NMR (300 MHz, CDCl3): δ 7.55 (dd, J = 19.3, 7.9 Hz, 2H), 7.29–7.25 (m, 1H), 7.04 (t, J = 7.6 Hz, 1H), 3.59–3.58 (m, 4H), 1.68 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 168.8, 152.8, 125.7, 120.9, 120.4, 118.6, 49.5, 24.1, 25.2; ESI-MS: m/z 219 [M + 1].2-(Piperidin-1-yl)benzo[d]oxazole (3j)
1H NMR (300 MHz, CDCl3): δ 7.51 (dd, J = 15.8, 7.5 Hz, 2H, 2H), 7.22 (t, J = 7.5 Hz, 1H) 7.03–6.96 (m, 1H), 3.59–3.51 (m, 4H), 1.69 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 161.7, 148.2, 142.0, 123.9, 122.0, 120.4, 115.5, 109.8, 108.5, 46.5, 25.0, 23.7; ESI-MS: m/z 203 [M + 1].2-(Pyrrolidin-1-yl)benzo[d]thiazole (3k)
1H NMR (300 MHz, CDCl3): δ 7.58 (dd, J = 8.3, 4.9 Hz, 1H), 7.31–7.24 (m, 1H), 7.06–7.02 (m, 1H), 3.58 (t, J = 6.6 Hz, 2H), 2.09–2.05 (m, 2H); ESI-MS: m/z 205 [M + 1].4-(Benzo[d]thiazol-2-yl)morpholine (3m)
1H NMR (300 MHz, CDCl3): δ 7.33–7.28 (m, 1H), 7.21–7.18 (m, 1H), 7.14–7.08 (m, 1H), 7.0–6.94 (m, 1H) 3.77–3.71 (m, 4H) 3.65–3.62 (m, 4H); 13C NMR (75 MHz, CDCl3): δ 168.9, 152.3, 126.0, 121.6, 120.7, 119.2, 66.1, 48.4; ESI-MS: m/z 221 [M + 1].2-Morpholinobenzo[d]oxazole (3n)
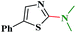
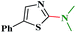
1H NMR (300 MHz, CDCl3): δ 7.60 (d, J = 7.7 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.32–7.28 (m, 1H), 7.12–7.07 (m, 1H), 3.83–3.81 (m, 4H), 3.62–3.60 (m, 4H); 13C NMR (75 MHz, CDCl3): δ 161.1, 148.1, 124.3, 123.5, 121.2, 108.8, 116.0, 65.9, 45.7; ESI-MS: m/z 205 [M + 1].N,N-Dimethyl-5-phenylthiazol-2-amine (3o)
1H NMR (300 MHz, CDCl3): δ 7.73 (d, J = 7.5 Hz, 2H), 7.23–7.08 (m, 3H), 6.52 (s, 1H), 2.90 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 151.8, 135.0, 128.2, 127.2, 125.8, 100.7, 39.8; ESI-MS: m/z 205 [M + 1].2-(4-Methylpiperazin-1-yl)benzo[d]oxazole (5m)
1H NMR (300 MHz, CDCl3): δ 7.58–7.47 (m, 2H), 7.27 (t, J = 7.7 Hz, 1H), 7.05 (t, J = 7.7 Hz, 1H), 3.63 (t, J = 4.9 Hz, 4H), 2.49 (t, J = 4.9 Hz, 4H), 2.31 (s, 3H); ESI-MS: m/z 286 [M + 1].2-(4-Ethylpiperazin-1-yl)benzo[d]thiazole (5n)
1H NMR (300 MHz, CDCl3): δ 7.60–7.54 (m, 2H), 7.29 (t, J = 7.7 Hz, 1H), 7.07 (t, J = 7.7 Hz, 1H), 3.66 (t, J = 5.0 Hz, 4H), 2.57 (t, J = 4.6 Hz, 4H), 2.50–2.45 (m, 2H), 1.12 (t, J = 7.1 Hz, 3H); ESI-MS: m/z 248 [M + 1].2-(4-Phenylpiperazin-1-yl)benzo[d]thiazole (5p)
1H NMR (300 MHz, CDCl3): δ 7.37–7.29 (m, 1H), 7.27–7.18 (m, 3H), 7.17–7.11 (m, 1H), 7.01–6.83 (m, 1H), 3.85 (t, J = 5.2 Hz, 4H), 3.28 (t, J = 5.2 Hz, 4H); 13C NMR (75 MHz, CDCl3): δ 161.8, 150.8, 148.6, 142.5, 129.2, 124.0, 120.9, 120.8, 116.9, 116.2, 108.7, 49.1, 45.5; ESI-MS: m/z 296 [M + 1].tert-Butyl-4-(benzo[d]oxazol-2-yl)piperazine-1-carboxylate (5v)
1H NMR (300 MHz, CDCl3): δ 7.35 (d, J = 7.5 Hz, 1H), 7.25 (d, J = 7.9 Hz, 1H), 7.17 (t, J = 7.4 Hz, 1H), 7.03 (t, J = 7.5 Hz, 1H), 3.56 (d, J = 7.5 Hz, 8H), 1.49 (s, 9H); 13C NMR (75 MHz, CDCl3): δ 161.7, 154.3, 148.5, 142.6, 123.9, 120.7, 116.2, 108.6, 80.1, 45.2, 28.1; ESI-MS: m/z 304 [M + 1].Acknowledgements
The authors thank the Council of Scientific and Industrial Research (CSIR), New Delhi for financial assistance.References
- (a) R. Hili and A. K. Yudin, Nat. Chem. Biol., 2006, 2, 284 CrossRef CAS PubMed; (b) Amino Group Chemistry, From Synthesis to the Life Sciences, ed. A. Ricci, Wiley-VCH, Weinheim, 2007 Search PubMed.
- (a) F. Ullmann, Ber. Dtsch. Chem. Ges., 1903, 36, 2382 CrossRef; (b) J. D. Senra, L. C. S. Aguiar and A. B. C. Simas, Curr. Org. Synth., 2011, 8, 53 CrossRef CAS; (c) Y. Jiang and D. Ma, in Catalysis without Precious Metals, ed. M. Bullock, Wiley-Blackwell, Weinheim, Germany, 2010, p. 213 Search PubMed; (d) L. Penn and D. Gelman, Chemistry of Organocopper Compounds, John Wiley & Sons, Chichester, U.K., 2009, Part 2, p. 881 Search PubMed.
- (a) D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27 RSC; (b) J. F. Hartwig, S. Shekhar, Q. Shen and F. B. Landeros, in Chemistry of Anilines, ed. Z. Rapaport, John Wiley & Sons, New York, 2007, vol. 1, p. 455 Search PubMed.
- (a) M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machado, Chem. Rev., 2009, 109, 4140 CrossRef CAS PubMed; (b) D. Davyt and G. Serra, Mar. Drugs, 2010, 8, 2755 CrossRef CAS PubMed; (c) V. S. C. Veh and R. Iyengar, in Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Pergamon, Oxford, UK, 2008, vol. 4, p. 487 Search PubMed.
- (a) V. Klimesova, J. Koci, K. Waisser, J. Kaustova and U. Mçllmann, Eur. J. Med. Chem., 2009, 44, 2286 CrossRef CAS PubMed; (b) C. Sheng, H. Xu, W. Wang, Y. Cao, G. Dong, S. Wang, X. Che, H. Ji, Z. Miao, J. Yao and W. Zhang, Eur. J. Med. Chem., 2010, 45, 3531 CrossRef CAS PubMed.
- (a) K. G. Liu, J. R. Lo, T. A. Comery, G. M. Zhang, J. Y. Zhang, D. M. Kowal, D. L. Smith, E. H. Kerns, L. E. Schechter and A. J. Robichaud, Bioorg. Med. Chem. Lett., 2009, 19, 1115 CrossRef CAS PubMed; (b) C. J. O'Donnell, B. N. Rogers, B. S. Bronk, D. K. Bryce, J. W. Coe, K. K. Cook, A. J. Duplantier, E. Evrard, M. Hajos, W. E. Hoffmann, R. S. Hurst, N. Maklad, R. J. Mather, S. McLean, F. M. Nedza, B. T. ONeill, L. Peng, W. Qian, M. M. Rottas, S. B. Sands, A. W. Scmidt, A. V. Shrikhande, D. K. Spracklin, D. F. Wong, A. Zhang and L. J. Zhang, J. Med. Chem., 2010, 53, 1222 CrossRef PubMed.
- (a) M. E. McDonnell, M. D. Vera, B. E. Blass, J. C. Pelletier, R. C. King, C. F. Metzler, G. R. Smith, J. Wrobel, S. Chen, B. A. Wall and A. B. Reitz, Bioorg. Med. Chem., 2012, 20, 5642 CrossRef CAS PubMed; (b) B. C. Cheah, S. Vucic, A. V. Krishnan and M. C. Kiernan, Curr. Med. Chem., 2010, 17, 1942 CrossRef CAS.
- K. G. Liu, J. R. Lo, T. A. Comery, G. M. Zhang, J. Y. Zhang, D. M. Kowal, D. L. Smith, L. Di, E. H. Kerns, L. E. Schechter and A. J. Robichaud, Bioorg. Med. Chem. Lett., 2009, 19, 1115 CrossRef CAS PubMed.
- J. Van Heudsen, R. Van Ginckel, H. Bruwiere, P. Moelans, B. Janssen, W. Floren, B. J. Van der Leede, J. van Dun, G. Sanz, M. Venet, L. Dillen, C. Van Hove, G. Willemsens, M. Janicot and W. Wouters, Br. J. Cancer, 2002, 86, 605 CrossRef PubMed.
- S. Massari, D. Daelemans, M. L. Barreca, A. Knezevich, S. Sabatini, V. Cecchetti, A. Marcello, C. Pannecouque and O. Tabarrini, J. Med. Chem., 2010, 53, 641 CrossRef CAS PubMed.
- (a) C. W. Cheung, D. S. Surry and S. L. Buchwald, Org. Lett., 2013, 15, 14 CrossRef PubMed; (b) L. L. Joyce and R. A. Batey, Org. Lett., 2009, 11, 13 CrossRef PubMed; (c) S. Toulot, T. Heinrich and F. R. Lerouxa, Adv. Synth. Catal., 2013, 355, 3263 CrossRef CAS; (d) S. Chen, K. Zheng and F. Chen, Tetrahedron Lett., 2012, 53, 6297 CrossRef CAS PubMed.
- (a) D. Ma, X. Lu, L. Shi, H. Zhang, Y. Jiang and X. Liu, Angew. Chem., Int. Ed., 2011, 50, 1118 CrossRef CAS PubMed; (b) S. K. Verma, B. N. Acharya and M. P. Kaushik, Org. Biomol. Chem., 2011, 9, 1324 RSC; (c) S. K. Rout, S. Guin, J. Nath and B. K. Patel, Green Chem., 2012, 14, 2491 RSC; (d) Y. Li, Y. Xie, R. Zhang, K. Jin, X. Wang and C. Duan, J. Org. Chem., 2011, 76, 5444 CrossRef CAS PubMed; (e) T. Kawano, K. Hirano, T. Satoh and M. Miura, J. Am. Chem. Soc., 2010, 132, 6900 CrossRef CAS PubMed; (f) X. Deng, H. McAllister and N. S. Mani, J. Org. Chem., 2009, 74, 5742 CrossRef CAS PubMed; (g) G. W. Stewart, C. A. Baxter, E. Cleator and F. J. Sheen, J. Org. Chem., 2009, 74, 3229 CrossRef CAS PubMed; (h) S. Guo, B. Qian, Y. Xie, C. Xia and H. Huang, Org. Lett., 2011, 13, 3 Search PubMed; (i) Y. Xie, B. Qian, P. Xie and H. Huanga, Adv. Synth. Catal., 2013, 35, 1315 CrossRef; (j) S. Chen, K. Zheng and F. Chen, Tetrahedron Lett., 2012, 53, 6297 CrossRef CAS PubMed.
- J. Wang, J. T. Hou, J. Wen, J. Zhang and X. Q. Yu, Chem. Commun., 2011, 47, 3652 RSC.
- (a) C. W. Cheung, D. S. Surry and S. L. Buchwald, Org. Lett., 2013, 15, 14 CrossRef PubMed; (b) A. R. Martin, Y. Makida, S. Meiries, A. M. Z. Slawin and S. P. Nolan, Organometallics, 2013, 32, 6265 CrossRef CAS.
- S. H. Cho, J. Y. Kim, S. Y. Lee and S. Chang, Angew. Chem., Int. Ed., 2009, 48, 9127 CrossRef CAS PubMed.
- S. Wertz, S. Kodama and A. Studer, Angew. Chem., Int. Ed., 2011, 50, 11511 CrossRef CAS PubMed.
- (a) J. Zhao, H. Huang, W. Wu, H. Chen and H. Jiang, J. Med. Chem., 2010, 53, 641 CrossRef PubMed; (b) M. Lamani and K. R. Prabhu, J. Org. Chem., 2011, 76, 7938 CrossRef CAS PubMed; (c) A. I. Khalaf, R. G. Alvarez, C. J. Suckling and R. D. Waigh, Tetrahedron, 2000, 56, 8567 CrossRef CAS; (d) T. P. Petersen, A. F. Larsen, A. Ritzén and T. Ulven, J. Org. Chem., 2013, 78, 4190 CrossRef CAS PubMed; (e) Y. S. Wagh, D. N. Sawant and B. M. Bhanage, Tetrahedron Lett., 2012, 53, 3482 CrossRef CAS PubMed.
- G. Satish, K. H. V. Reddy, K. Ramesh and K. Karnakar, Tetrahedron Lett., 2012, 53, 2518 CrossRef CAS PubMed.
- (a) K. H. V. Reddy, G. Satish, V. P. Reddy, B. S. P. A. Kumar and Y. V. D. Nageswar, RSC Adv., 2012, 2, 11084 RSC; (b) A. V. Kumar, V. P. Reddy, R. Sridhar, B. Srinivas and K. R. Rao, Synlett, 2009, 5, 739 Search PubMed; (c) A. V. Kumar, V. P. Reddy and K. R. Rao, Synlett, 2010, 17, 2571 Search PubMed; (d) T. M. Gadda, X. Y. Yu and A. Miyazawa, Tetrahedron Lett., 2010, 66, 1249 CrossRef PubMed.
- (a) K. Swapna, S. N. Murthy, M. T. Jyothi and Y. V. D. Nageswar, Org. Biomol. Chem., 2011, 9, 5978 RSC; (b) K. Swapna, S. N. Murthy, M. T. Jyothi and Y. V. D. Nageswar, Org. Biomol. Chem., 2011, 9, 5989 RSC; (c) K. H. V. Reddy, V. P. Reddy, J. Shankar, B. Madhav and Y. V. D. Nageswar, Tetrahedron Lett., 2011, 52, 2679 CrossRef CAS PubMed; (d) K. H. V. Reddy, V. P. Reddy, J. Shankar and Y. V. D. Nageswar, Synlett, 2011, 9, 1268 Search PubMed; (e) K. H. V. Reddy, A. A. Kumar, G. Kranthi and Y. V. D. Nageswar, Beilstein J. Org. Chem., 2011, 7, 886 CrossRef CAS PubMed; (f) K. H. V. Reddy, G. Satish, K. Ramesh, K. Karnakar and Y. V. D. Nageswar, Chem. Lett., 2012, 41, 585 CrossRef CAS; (g) B. S. P. A. Kumar, K. H. V. Reddy, B. Madhav, K. Ramesh and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 4595 CrossRef PubMed; (h) K. H. V. Reddy, G. Satish, K. Ramesh, K. Karnakar and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 3061 CrossRef CAS PubMed; (i) G. Satish, K. H. V. Reddy, K. Ramesh and B. S. P. A. Kumar, Tetrahedron Lett., 2014, 55, 2596 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05447d |
| This journal is © The Royal Society of Chemistry 2014 |